A carboxy-methyl cellulose carrier reduces bone formation within a silicate-substituted calcium phosphate scaffold
-
1
University College London, Institute of Orthopaedics and Musculoskeletal Science, United Kingdom
-
2
ApaTech, Research and Development, United Kingdom
Introduction: Recently, injectable and moldable forms of bone substitute material, such as pastes and putties, have been developed as they offer many advantages over dry granules including increased handling ability and are able to completely fill contained defects of complex geometric shapes. This study investigated the effect of using carboxy-methyl cellulose (CMC) as the binding agent on bone formation within a porous silicate-substituted calcium phosphate (SiCaP) scaffold implanted in an ovine femoral condyle critical-sized defect. Our hypothesis was that CMC would have no negative effect on bone formation and osteoconduction within the scaffold.
Methods: Twenty-four 8x15mm deep defects were created in the medial femoral condyles of 6 sheep. Defects were (1) empty, or filled with either (2) SiCaP granules, (3) SiCaP Putty or (4) a SiCaP press-fit block. Implants remained in vivo for 4, 8 and 12 weeks (n=6). Scaffolds in each group were identical in composition and consisted of a phase pure SiCaP (0.8 wt % Si) with a total macroporosity of 80%, a microporosity of 22.5% and mean pore size of 300 μm. The SiCaP granules used in groups 2 and 3 were identical and the groups differed only by the addition of CMC. Fluorochrome markers were used to measure bone apposition rates and following retrieval, specimens were processed for histology. A thin section was made through the centre of each defect and image analysis techniques used to quantify bone apposition rates, bone area, bone-implant contact and implant area.
Results: No new bone growth was measured within any of the empty defects. At 4 weeks, greatest bone apposition rates were measured in the SiCaP granules group (1.02±0.49μm/day-1) (figure 1)
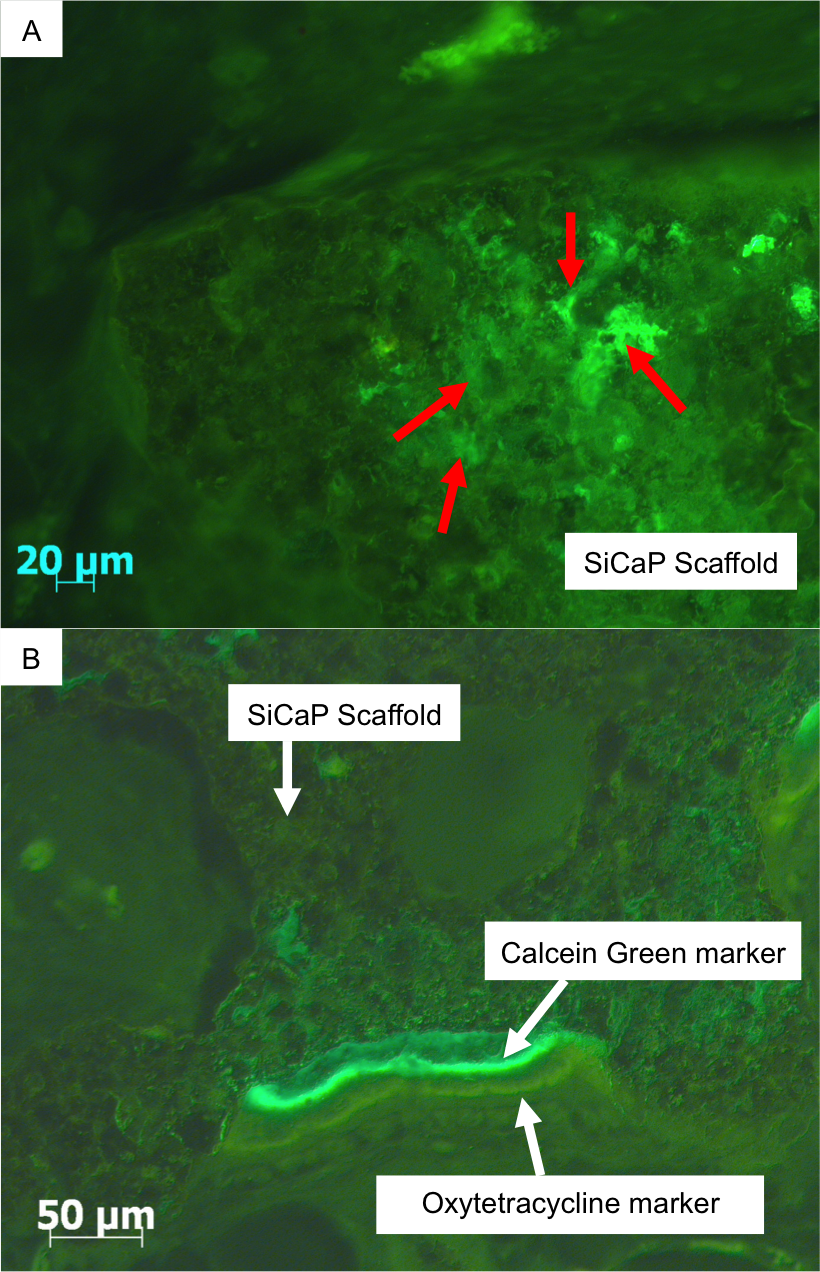
. However, by 12 weeks no significant differences were observed between groups.
At 4 weeks significantly increased bone was measured adjacent to SiCaP granules (3.97±1.11%) when compared with both SiCaP Putty (0.09±0.06%; p = 0.028) and SiCaP block scaffolds (0.15±0.08%; p=0.028) (Figure 2)
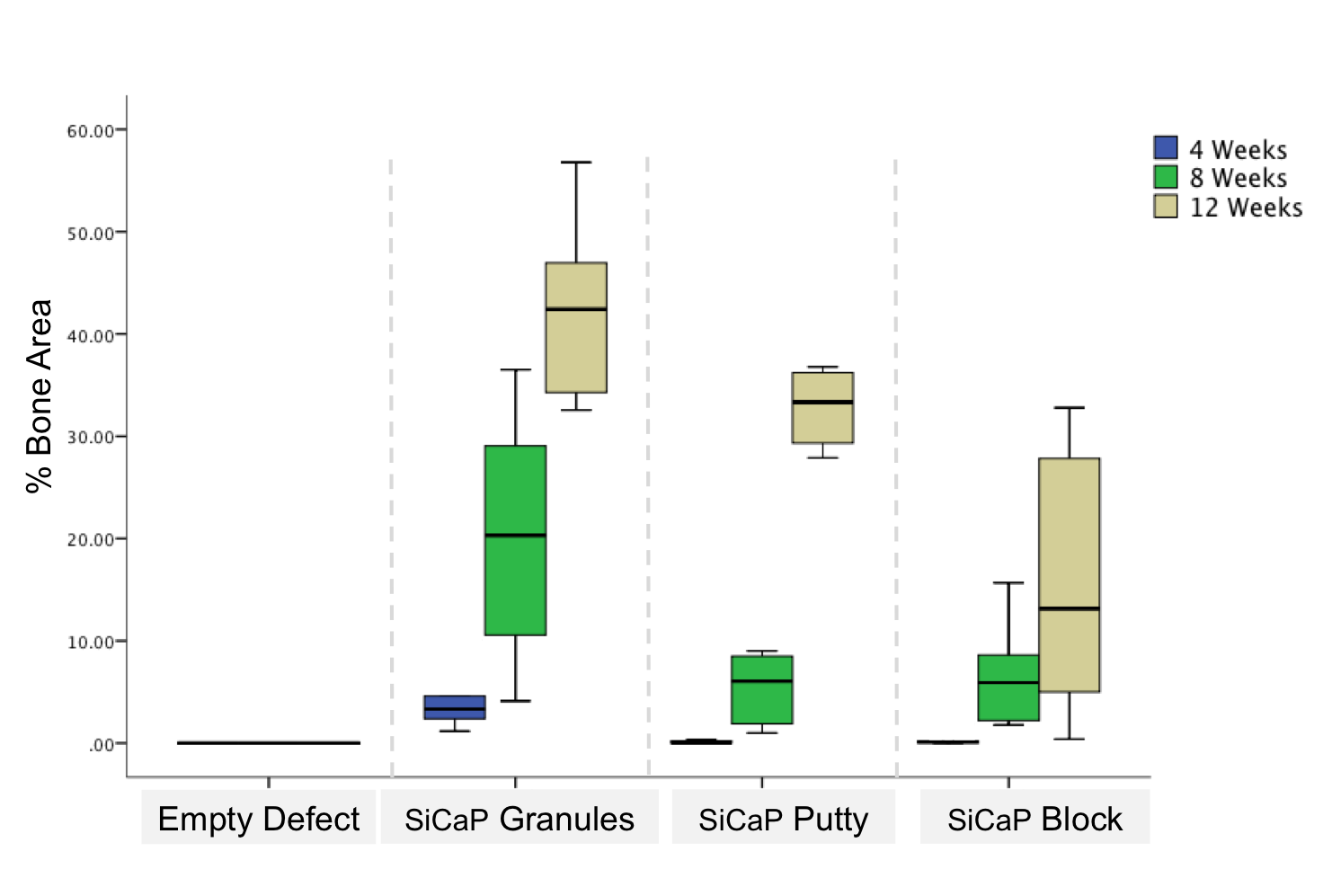
. At 12 weeks, significantly more bone was measured in the SiCaP granules group (40.56±3.73%) when compared with SiCaP blocks (15.38±5.30%; p=0.028). Longitudinal analysis showed that in all groups, apart from the empty defect, bone area significantly increased from 4 to 12 weeks.
At 4 weeks, significantly increased bone-implant contact was seen in the SiCaP granules group (10.25±3.05%) when compared with both the SiCaP Putty (0.63±0.63% p=0.028) and SiCaP block group (1.05±0.45%; p=0.028) (Figure 3)
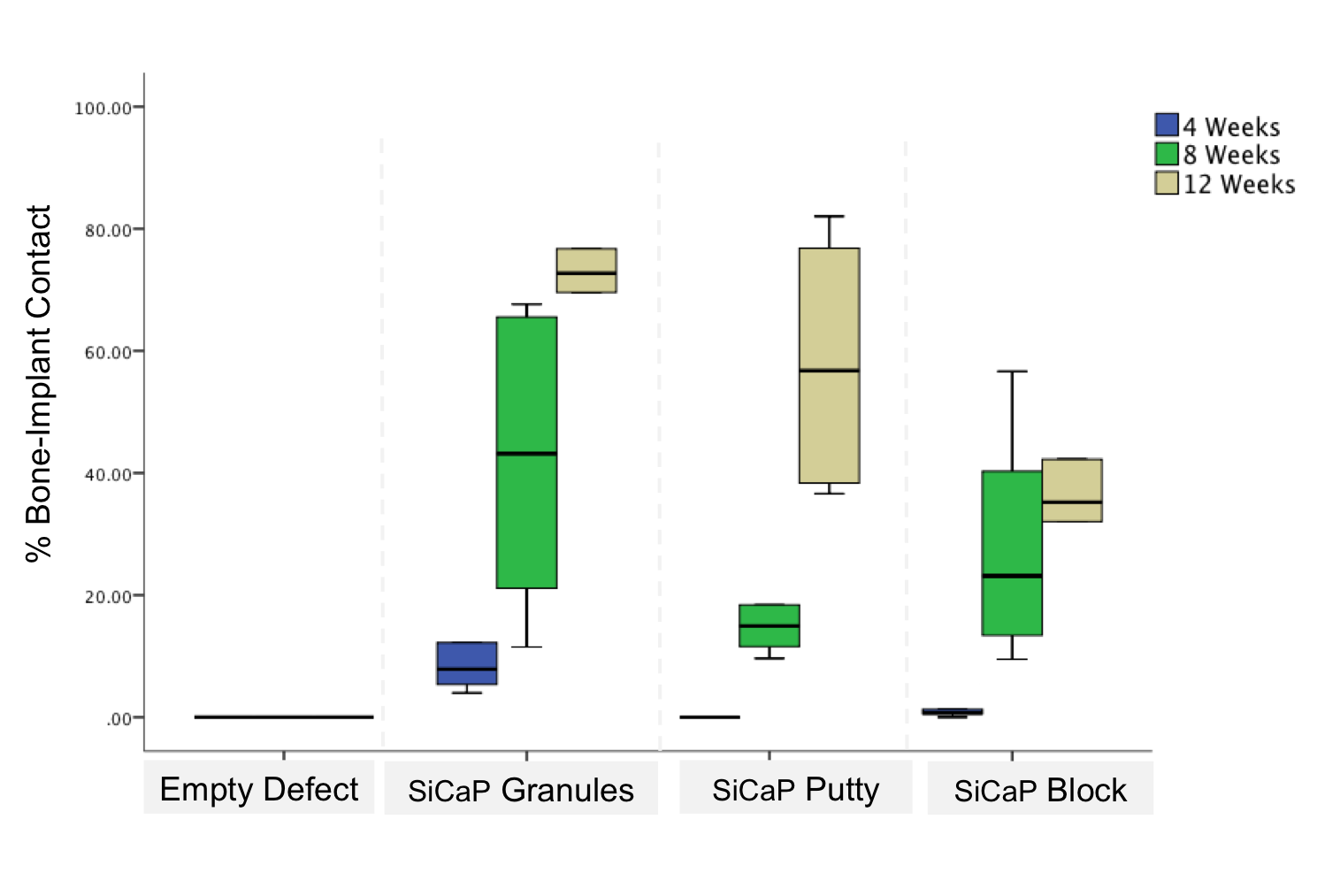
. At 12 weeks significantly increased bone-implant contact was measured in the SiCaP granules group (70.84±6.66%) when compared with the SiCaP block specimens (37.53±7.73%; p=0.028). Longitudinal analysis showed that in all groups, significantly increased amounts of bone-implant contact was measured.
Results showed implant area gradually decreased in all groups over time and no significant differences was observed. The highest rate of implant resoprtion was measured in the SiCaP granule group.
Discussion: CaP granules significantly increased bone formation when compared with a press-fit block composed of the same material. This may be due to the increased surface area associated with granules and subsequent increased scaffold resorption. Results also showed the detrimental effect that CMC has on bone growth and osteoconduction.
Keywords:
Bone Regeneration,
Calcium phosphate,
Bone graft,
Biodegradable material
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials evaluation in animal models
Citation:
Coathup
M,
Campion
C and
Blunn
GW
(2016). A carboxy-methyl cellulose carrier reduces bone formation within a silicate-substituted calcium phosphate scaffold.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00150
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.