Introduction: Stem cell geometry has been linked to differentiation[1],[2]. We hypothesize that the effects of geometric confinement on stem cells is time dependent. To study this hypothesis, we developed intrinsically degradable protein patterns. Briefly, alkanethiol self-assembled monolayer (SAM) patterns were deposited on gold (Au) of various thicknesses. Fibronectin was then adsorbed to these patterns (Fig 1A). Degradation of these surfaces was tested in various physiological environments (e.g. cell culture media) and characterized using physical and chemical methods. Differentiation of human mesenchymal stem cells (HMSCs) on these surfaces was investigated.
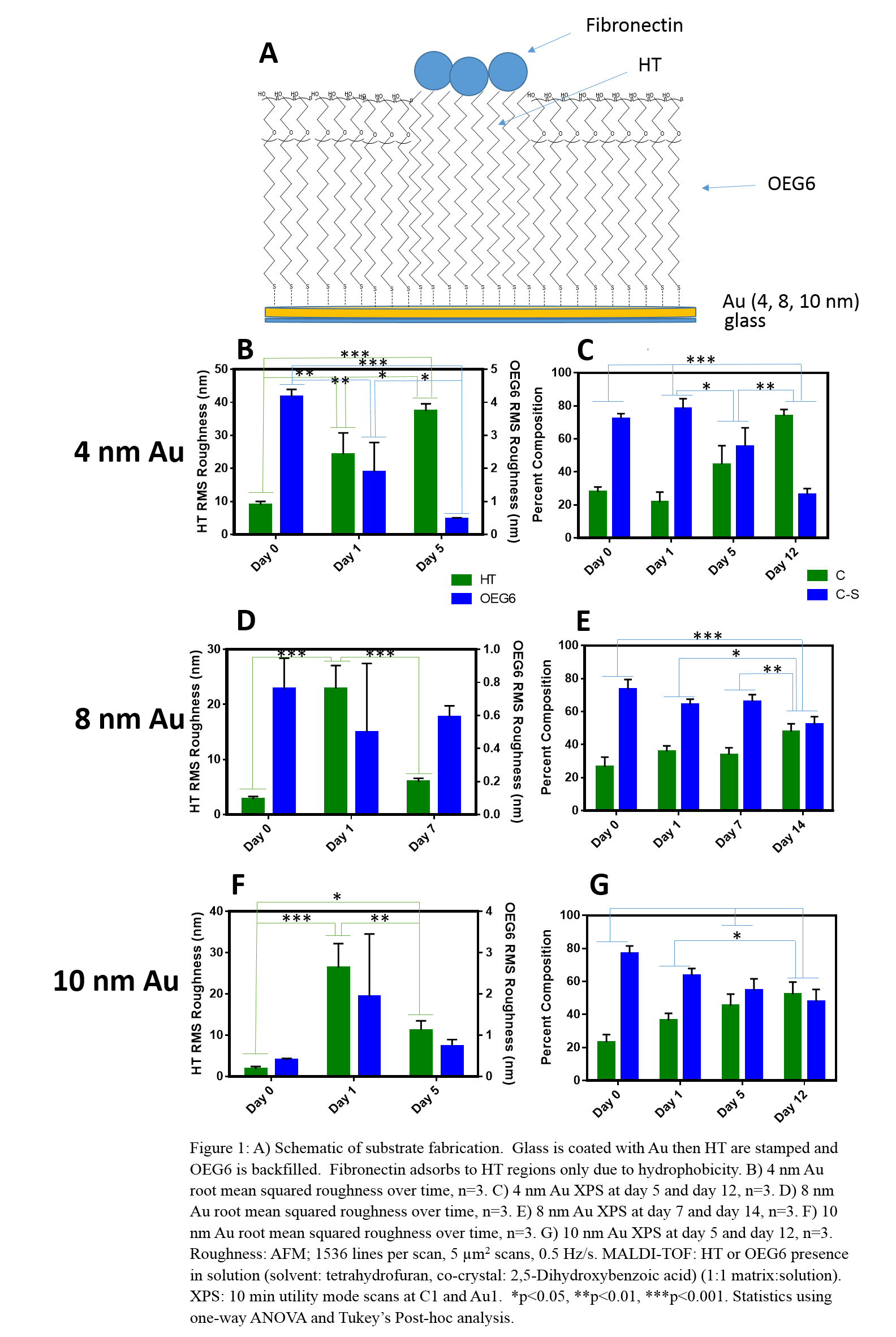
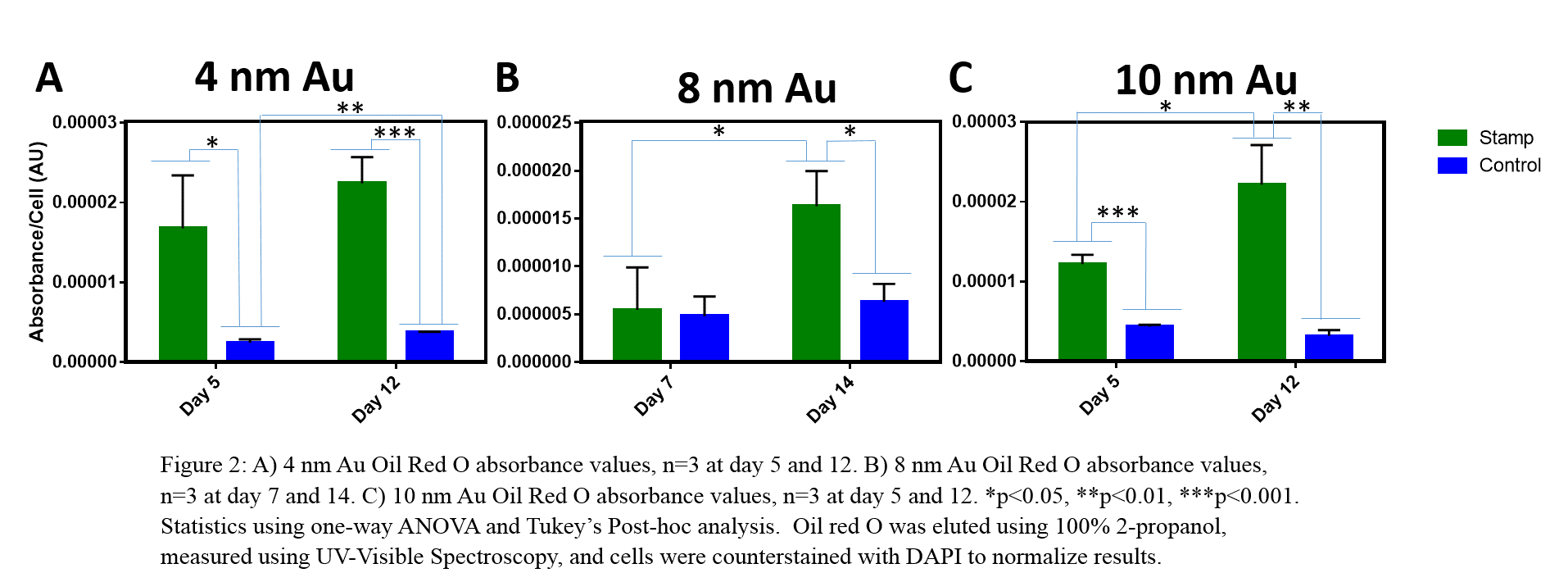
Methods: Fabrication and Characterization: Hexadecanethiol (HT) and 2-{2-[2-(2-{2-[2-(1-mercaptoundec-11-yloxy)-ethoxy]-ethoxy}-ethoxy)-ethoxy]-ethoxy}-ethanol thiols (OEG6) (4 mM) were applied to Au-coated glass. Characterization methods included atomic force microscopy (AFM), matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry, and x-ray photoelectron spectroscopy. Cell Culture and Differentiation: HMSCs were cultured in 1:1 v/v osteogenic:adipogenic differentiation media. Lipids were stained with oil red O, and calcification was measured by an alkaline phosphatase activity assay.
Results and Discussion: We examined the physical changes of our SAM surfaces in cell culture media to understand behavior in cell culture conditions. An increase in roughness 24 hours after HT deposition indicates protein adsorption. At day 5, 4 nm Au showed increased roughness (Fig 1B), suggesting Au mottling[3]. HT-Au, 8 nm (Fig 1D) and 10 nm (Fig 1F), show a decrease in roughness over time, suggesting desorption of HT and proteins likely due to metal oxidation. Au thickness affected stability; 8 and 10 nm Au showed greater stability than 4 nm Au. We examined the chemical changes associated with degradation to confirm that the mechanism of degradation was Au oxidation and mottling. MALDI-TOF peaks for thiol, glycol, and Au fragments were seen in 4, 8, and 10 nm Au-OEG6 on days 5, 7, and 5, respectively, further indicating Au mottling and OEG6 desorption. XPS results demonstrated a decrease of carbon-sulfur and increase in carbon over time (Fig 1C,E,G), while the total Au did not change, indicating that degradation was driven primarily by oxidation. Finally, cells confined by these substrates demonstrated increased levels of lipid formation as compared to non-confined controls (Fig 2), which continued to increase over time as the patterned surfaces underwent degradation for all Au thicknesses examined; there was no change in calcification.
Conclusions: The degradation behavior of these Au-SAM surfaces may be used in stem cell engineering. Our studies confirmed that Au oxidation and mottling cause pattern degradation, dependent on Au thickness. Confinement on our substrates affected stem cell differentiation in a time dependent manner.
Shukla Laboratory
References:
[1] Kilian, K. a, Bugarija, B., Lahn, B. T., & Mrksich, M. (2010). Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America, 107(11), 4872–7. http://doi.org/10.1073/pnas.0903269107
[2] Mrksich, M., Dike, L. E., Tien, J., Ingber, D. E., & Whitesides, G. M. (1997). Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Experimental Cell Research, 235(2), 305–13. http://doi.org/10.1006/excr.1997.3668
[3] Ostuni, E., et al., Using self-assembled monolayers to pattern ECM proteins and cells on substrates. Methods Mol Biol, 2009. 522: p. 183-94.