Hydrogels have received considerable attention in the context of soft tissue engineering due to their as high water contents, (general) cell compatibility, and physicochemical and mechanical similarities to native soft tissues[1]. Most hydrogels used for such applications are either monolithic or macroporous with primarily spherical pore structures, despite the fact that it is known that native extracellular matrix (ECM) contains primarily fibrous structures that play key roles in regulating cell behavior[2],[3]. Consequently, to mimic these fibrous ECM gel structures using a single processing step, we aim to prepare crosslinked fibrous hydrogels in situ via electrospinning of reactive functional precursor polymers.
Poly(oligoethylene glycol methacrylate) (POEGMA) was functionalized with hydrazide and aldehyde groups via copolymerization; when mixed, these functionalized polymers crosslink rapidly to form hydrazone bonds[4]. POEGMA precursors (Mw ~ 30 kDa, 15 wt% in deionized water) and PEO (Mw = 600,000 g/mol, 3-5 wt%) were loaded into separate barrels of a double-barrel syringe mounted on a syringe pump. A voltage of 8.5 kV was provided to a conductive needle (18G), with electrospinning conducted using a 10 cm falling distance between the needle and collector.
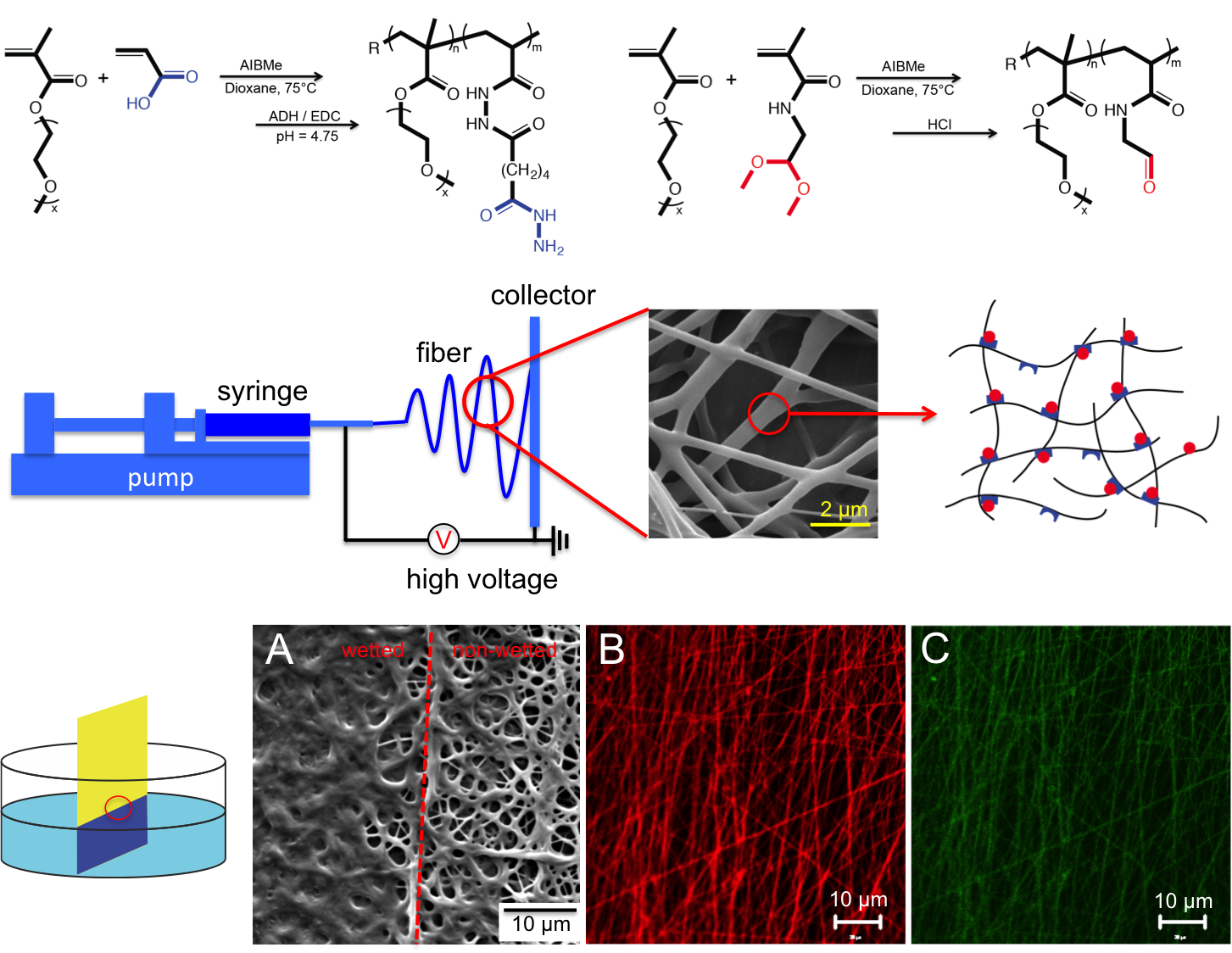
POEGMA could not be effectively electrospun directly due to its low molecular weight (~30 kDa) and low entanglement; as such, PEO was used as an electrospinning aid. Continuous nanofibers with an average diameter of 340∓80 nm were obtained. By changing mass ratio of POEGMA to PEO from 3:1 to 10:1 or 15:1, the continuous nanofibrous structure was transformed to bead-on-fiber and finally to a bead-only structure. When any of these scaffolds was exposed to water, a clear increase in nanofiber diameter was observed; however, unlike PEO nanofibers that dissolve fully within minutes, the POEGMA scaffold remains stable. Differential scanning calorimetry data indicated that PEO could be removed nearly quantitatively from the hydrogel fibers following production by simple soaking in water. FTIR-ATR confirmed the presence of POEGMA in the nanofibers, while labeling of the hydrazide POEGMA polymer with fluorescein and the aldehyde POEGMA polymer with rhodamine confirmed co-localization of the reactive precursor polymers throughout the nanofibers. The scaffolds quickly hydrated and swelled upon exposure to 10 mM PBS, achieving the maximum water content of ~92% in less than one minute; in contrast, a bulk hydrogel of the same composition required nearly two hours to reach equilibrium swelling. Incubation of the electrospun scaffold in 1M HCl results in complete dissolution of the network within ~100 hours, while scaffold stability in water was maintained for 8-10 weeks prior to hydrolysis of the hydrazone crosslinks. All the matrices have mechanical stability both in the dry state as well as when swollen in PBS under at least 20% compression.
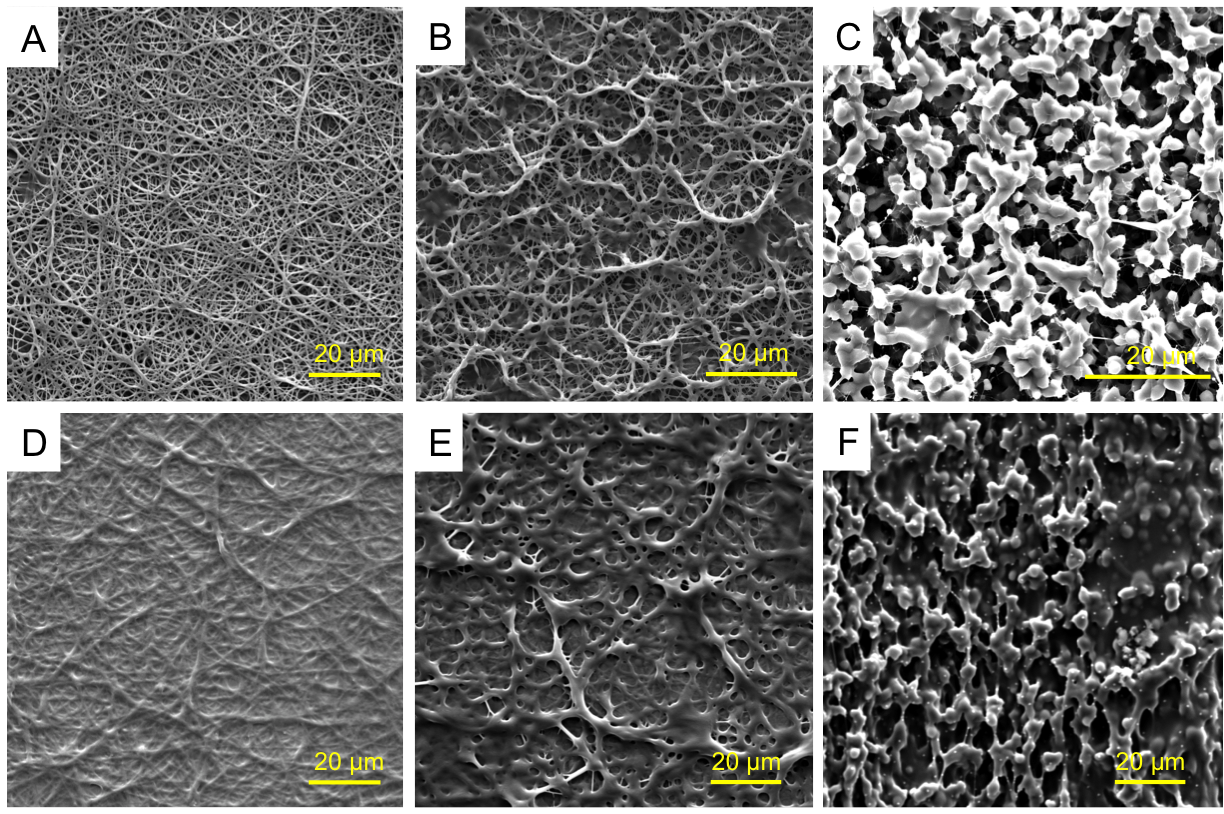
Nanofibrous hydrogels based on POEGMA were fabricated via reactive electrospinning. The scaffold morphology can be facilely controlled from fibers to beads by changing the PEO concentration without compromising the coherence of the hydrogel film produced. Consequently, we anticipate these matrices have significant potential in multiple cell scaffolding or adhesion barrier applications.
Funding from the Natural Sciences and Engineering Research Council of Canada and the NSERC CREATE-IDEM (Integrated Development of Extracellular Matrices) training program is gratefully acknowledged.; José Moran-Mirabal is thanked for use of his electrospinning equipment and helpful advice.
References:
[1] D. Seliktar, "Designing cell-compatible hydrogels for biomedical applications," Science. Vol. 336, Jun. 2012.
[2] M. M. Stevens and J. H. George, "Exploring and engineering the cell surface interface," Science. Vol. 310, Nov. 2005.
[3] C. He, W. Nie and W. Feng, "Engineering of biomimetic nanofibrous matrices for drug delivery and tissue engineering," J. Mater. Chem. B. Vol. 2, Sep. 2014.
[4] N. M. B. Smeets, E. Bakaic, M. Patenaude and T. Hoare, "Injectable and Tunable Poly(ethylene glycol)-Analogue Hydrogels Based on Poly(oligoethylene glycol methacrylate)," Chem. Commun. Vol. 50, Jan. 2014.