Introduction: Spinal fusion is one of the most common surgeries performed worldwide. In fact, the forecasted global spine surgery device market is estimated to be worth $14.8 billion by 2017. Despite robust market growth and nearly two decades of development, clinical studies still demonstrate that the function of synthetic spinal fusion devices is predominately mechanical and there remains a critical need to improve the potential of these devices[1] to meet the demanding biological performance standards to integrate with and repair bony tissues[2]. Bone integration with an interbody implant will aid fusion and improve implant longevity by limiting subsidence, stress shielding and associated complications[1]. To achieve this goal, it is essential to incorporate cues into spinal fusion devices that facilitate osteopromotion, i.e., enhancement of endogenous cell ongrowth and osteogenic differentiation.
Osteoblasts are the primary bone-forming cell in the body and their precursor, the mesenchymal stem cell (MSC), constitutes an endogenous source of cells for enhancing spinal fusion outcomes. Micropatterns have been shown to influence the orientation and polarization (i.e., elongation) of cells by controlling placement of focal adhesions through a phenomenon known as contact guidance. Contact guidance is a key regulator of both cellular migration and differentiation[3],[4]. Based on these findings we hypothesized that Sharklet micropatterns that increased cellular anisotropy would promote cell migration and osteogenesis[4].
Methods: The samples depicted in Fig. 1 represent different variations of the Sharklet micropattern, where rectangular features of varying lengths (4 to 80 μm) make up a diamond pattern that repeats across the surface. These samples were created in poly(dimethylsiloxane) elastomer (PDMSe) with features protruding from the surface at a height of ~2 μm. The width and spacing of the features were 2 μm x 2 μm or 10 μm x 5 μm.
To investigate the influence of Sharklet micropatterns on cell morphology, samples of two micropattern configurations and smooth (SM) controls were treated with fibronectin (15 μg/ml, overnight), and then MSCs were cultured on these samples for 3 d in growth media (low-glucose Dulbecco’s modified eagle medium (DMEM) (Gibco) supplemented with 10% FBS (Invitrogen), 1 ng/ml recombinant human fibroblast growth factor-basic (FGF-2, Peprotech), 50 U/ml each penicillin/streptomycin (Gibco) and 1 μg/ml Fungizone antimycotic (Gibco)). Samples were subsequently fixed with 4% paraformaldehyde for 15 min at room temperature, stained with CellTracker according to the manufacturer’s instructions and imaged. Average cellular aspect ratios were measured using ImageJ software.
Histological staining for alkaline phosphatase (ALP), an early osteogenic marker, was performed at 7, 14 and 21 d to screen micropatterns for the promotion of osteogenesis. The intensity of ALP stain was scored for each cell and averaged over 3 replicates of 3 experiments and compared using ANOVA/Tukey’s test.
Results: Cell shape measurements show that MSCs elongated with Sharklet features, as measured by the average cellular aspect ratio (4.1 ± 0.1 on SM, 5.4 ± 0.12 on +1.7SK2x2 and 6.0 ± 0.14 on +1.5SK10x5). These data confirm that Sharklet micropatterns can be intentionally designed to modulate cell shape to aspect ratios that are significantly higher than those cultured on SM controls, which will regulate differentiation. ALP staining confirmed that MSCs cultured on the +1.7SK2x2 micropattern produced higher levels of ALP, 62% increase on Day 14 and 37% increase on Day 21 compared to SM controls (Fig. 1).
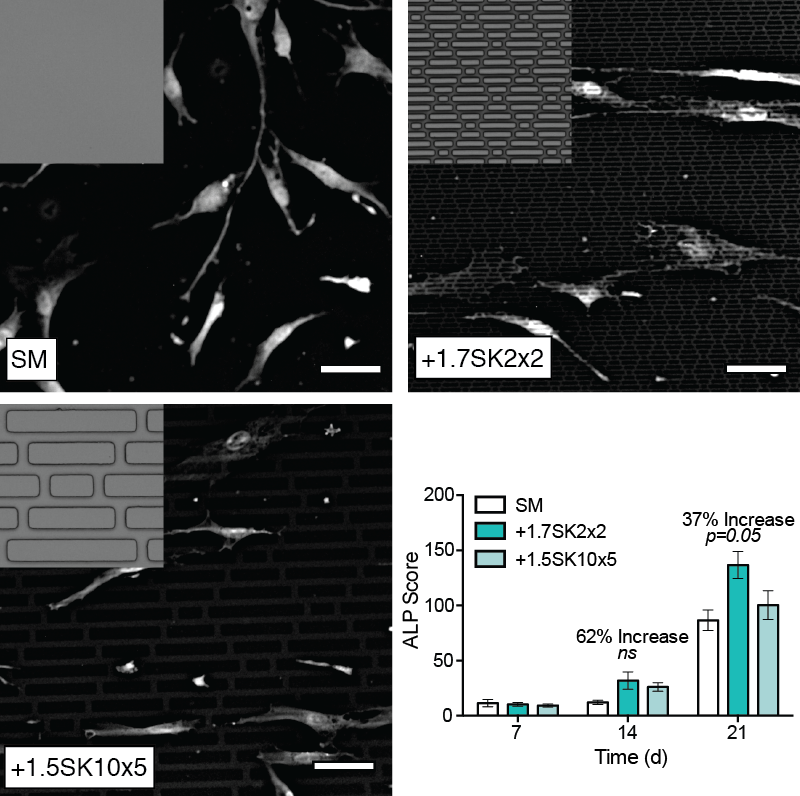
Fig. 1. Representative images of MSCs on test surfaces. Results show that Sharklet micropatterns precisely controlled cellular aspect ratio and increased ALP production compared to SM at D14 and D21.
Conclusions: Sharklet-patterned PDMSe induced higher average cellular aspect ratios and subsequently levels of ALP in MSCs cultured on the surface for up to 21 days versus SM controls. Collectively, these results provide the foundation for continued research to demonstrate the feasibility of using Sharklet micropatterned fusion devices to facilitate osteopromotion and enhance fusion outcomes.
References:
[1] P. J. Rao, et al., J Orthop Surg 2014, 6, 81.
[2] M. R. Abdullah, et al., J. Biomed. Mater. Res. 2015.
[3] M. Guvendiren, et al., Adv Healthc Mater 2012, 2, 155.
[4] K. A. Kilian, et, PNAS 2010, 107, 4872.