Brain injury results in neuronal loss and disruption of the brain parenchyma. However, the pathology of brain injury is not only dictated by the initial insult, but there is a significant secondary injury caused by a subsequent inflammatory response, which includes astrocytes becoming reactive and proliferating locally. Therefore, the current neurological problem is to attenuate the inflammatory response of the injured brain to promote the long-term survival of neurones and circuitry reconstruction. However, preventing secondary injury alone will not be adequate to promote brain repair, as neuroprotection within the penumbra will be required immediately following tissue insult with control over the inflammatory cascade promoting their long term survival. Ultimately, repair post brain injury will depend on: (a) restoring the architecture of damaged tissue; (b) transplanting replacement cells into the injury site; (c) providing a suitable microenvironment for transplanted cells to interegrate with the host and (d) to support surviving cells within the penumbra.
Fmoc-DDIKVAV was synthesised using solid-phase peptide synthesis[1]. Hydrogels were formed via a pH switch at a concentration of 10mg/ml and were characterised by Rheology, Fourier Transform Infrared Spectroscopy, Circular dichroism and Transmission Electron Microscopy confirmed the self-assembly of Fmoc-DDIKVAV[2]. The stroke model was achieved by stereotaxically inject human ET-1 peptide (800pmol in 1mL) into the frontal, motor cortex of rats to cause vasoconstriction and focal necrotic cell death. hESC due to it being a sustainable source, the ability to standardize the donor material (unlike fetally-derived tissue), availability of GFP+ reporter lines and relevance to future clinical application. We will differentiate GFP+ human embryonic stem cells (hESCs) into forebrain cortical progenitors. Differentiated GFP+ cells (100,000 cells in 1mL) will be grafted into the cystic lesion cavity. The grafts where characterised by assessing behaviour, cortical atrophy and using immunocytohistochemistry.
Here, we have shown that the trasplanting hESC derived neural progenitor cells (NPCs) concomidently with our Fmoc-DDIKVAV hydrogel across the necrotic lesion cavity, provided both physical and trophic support to transplanted cells, as well as for host cells withing the penumbra. The self-assembling peptide scaffolds were shown to significantly improve the survival and integration of the transplanted cells, along with a significant reduction in secondary degeneration caused by reactive astrocytosis. hESC derived NPCs that were transplanted within our Fmoc-DDIKVAV hydrogels, showed enhanced behavioural recovery along with increased functional maturity over a 9 month period, compared to cell grafts alone control group. While the focus of this study was on repairing the injured brain, our biologically relevant hydrogels have broader implications for the exploitation in areas such as cancer therapy, muscle and bone regeneration to name a few.
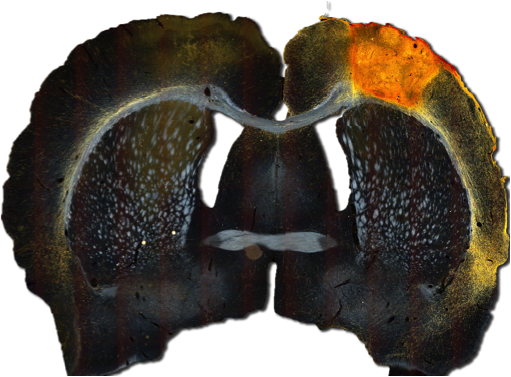
Fig. 1 - Shows the intergration and reinnervation of transplanted hESC dertived NPCs within a Fmoc-DDIKVAV hydrogel.
Funding for this research was obtained from the National Health and Medical Research Council, Australia (NHMRC, APP1050684), and the Australian Research Council (ARC, DP130103131). RJW was funded via an Alfred Deakin Research Fellowship; CLP was supported by Senior Medical Research Fellowship provided by the Viertel charitable Foundation, Australia; DRN was supported by an ARC Australian Postdoctoral Fellowship, and subsequently by a NHMRC Career Development Fellowship.
References:
[1] A. L. Rodriguez, C. L. Parish, D. R. Nisbet and R. J. Williams, Soft Matter, 2013, 9, 3915-3919.
[2] A. Rodriguez, T. Wang, K. Bruggeman, C. Horgan, R. Li, R. Williams, C. Parish and D. Nisbet, Journal of Materials Chemistry B, 2014, 2, 7771-7778.