Introduction: Injectable dermal fillers have recently been the subject of great interest for skin tissue augmentation[1]. Among commercial products, hyaluronic acid (HAc) is the most widely used natural polymer due to its biocompatibility and biodegradability. However, it often lasts for only 6 months in vivo. Furthermore, it as the sole substance lacks of promoting cell affinity to induce collagen formation[2]. Calcium phosphate (CaP), as a superior bioactive material, not only exhibits biocompatibility properties, along with natural degradation, but it also promotes tissue regeneration. In this study, we have developed HAc-CaP composite hydrogels to enhance both biostability and bioactivity for dermal filler application.
Methods: HAc hydrogel crosslinked by butanediol diglycidyl ether (BDDE) was fully swollen in distilled water. It was then immersed in the mixture of CaCl2 and H3PO4 for 2 h. Subsequently, it was dipped in 15 % NH4OH solution for 30 min to precipitate CaP within the hydrogel. The composite fillers with various CaP contents from 12.5 to 37.5 wt% were prepared as homogenizing them into particles at 7000 rpm for 5 min. Rheological performance was assessed by parallel plate rheometer, and in vivo volumetric analysis with time, compared against pure HAc fillers. In addition, histocompatibility after injection in the mouse’s skin was analyzed by Masson’s Trichrome (M&T) staining to verify collagen production.
Results: As shown in Fig.1A, all the composite fillers showed nano-roughened surface with the size of CaP from 110 to 190 nm, as compared to the pure HAc filler. The composite fillers showed significant enhancement on physical properties with higher elastic modulus against strain (Fig.1B). The axial force on the composite fillers drastically increased, compared to the pure HAc, indicating greater cohesivity and structural stability than pure HAc (Fig. 1C). The remaining volume ratio of the composite fillers was greater than that of the pure HAc fillers with longer in vivo durability as indicated in Fig.2. In addition, histological analysis with M&T staining revealed newly formed collagen around the composite filler, which were less observed in the pure HAc, as shown in Fig.3 (left). Larger portions of collagen fibers were also observed from TEM images of skin tissue on the composite filler, rather than on the pure HAc (Fig.3 right).
Conclusions: The aforementioned results suggest that the composite filler with nano CaP can have great potential for soft tissue augmentation with greater resistance under deformation and in vivo lifting capacity as well as better bioactivity than the pure HAc fillers.
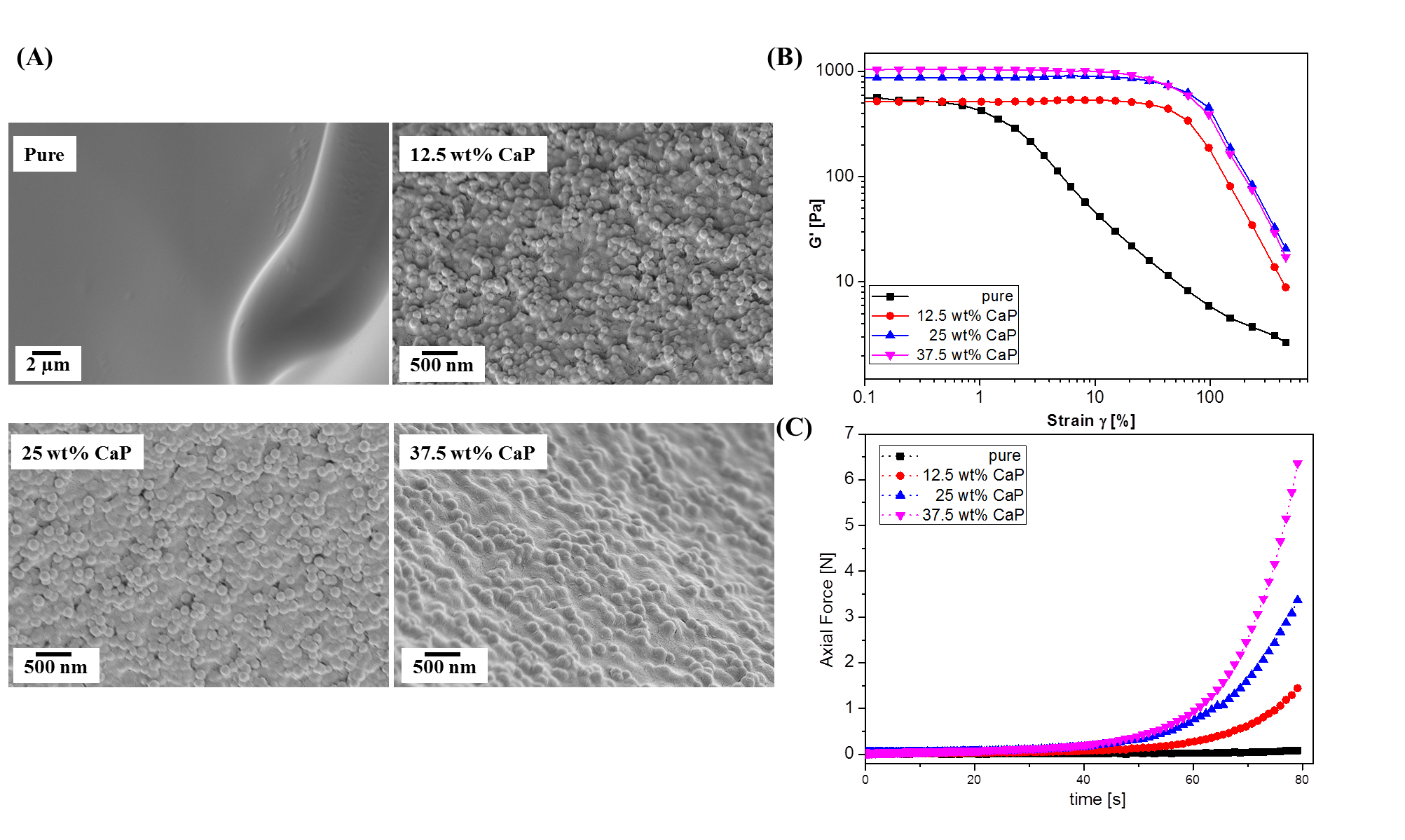
Figure 1. (A) Surface morphology, (B) Storage modulus at strain sweep and (C) Axial Force of hydrogel fillers with different CaP content

Figure 2. (A) Optic images of hydrogel fillers with different CaP content after 3 week injection in mouse skin (B) Remaining volume ratio after 1, 3 week implantation
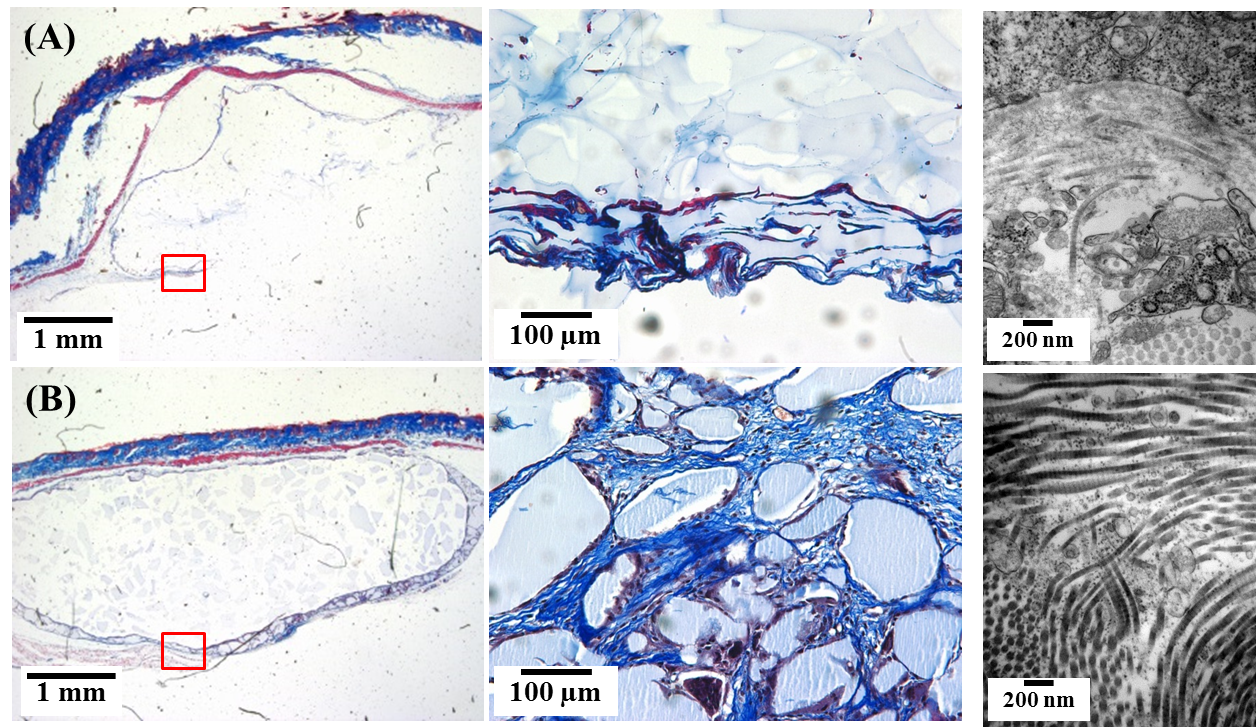
Figure 3. Histological images by M&T staining and TEM images of hydrogel fillers with (A) 0 and (B) 37.5 wt% CaP for regenerated collagen observation
This work was supported by the R&D Program (S2174750) funded by the Small and Medium Business Administration(SMBA, Korea)
References:
[1] Beasley KL, Weiss MA, Weiss RA., Facial Plast Surg. 25:86-94(2009)
[2] Alijotas-Reig J, Fernandez-Figueras MT, Puig L. Semin Arthritis Rheu. 43:241-58.(2013)