Magnetic Bacterial Nanocellulose for neurovascular reconstruction in cerebral aneurysm treatment applications
-
1
University of Illinois at Urbana-Champaign, Department of Bioengineering, United States
-
2
University of Illinois at Urbana-Champaign, Micro and Nanotechnology Laboratory, United States
-
3
University of Illinois at Urbana-Champaign, Department of Nuclear, Plasma and Radiological Engineering, United States
-
4
University of Antioquia, Department of Materials Engineering, Colombia
Introduction: Cerebral aneurysms are abnormal blood pockets that form outside of the arterial wall in the brain. The main obstacle in aneurysmal neuroendovascular reconstruction approaches is the delayed closure of the aneurysm due to poor cell retention[1]. We hypothesized that a magnetic bacterial nanocellulose (MBNC) can improve local cell retention at the site of the aneurysmal neck defect via focal magnetic attractive forces. Bacterial nanocellulose (BNC) is a natural hydrogel produced by A. Xylinum, which has high mechanical properties (114 GPa for a single filament of BNC)[2], and is biocompatible[3]. To test the feasibility of our approach, we precipitated iron oxide nanoparticles (IONPs) in BNC pellicles to yield a magnetic hydrogel, and characterized the resulting material via XPS, SEM and vibrating sample magnetometer (VSM). We additionally introduced cell adhesion sites (collagen) on the MBNC via 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) activation of the cellulose fibrils. A cytotoxicity test based on intracellular esterase activity was used to test the biocompatibility of the MBNC pellicles.
Materials and Methods: Magnetic functionalization: The MBNC was prepared by in-situ precipitation of Fe2+ and Fe3+ ferrous ions in never-dried BNC[4].
Collagen bio-conjugation: 100 ul of CDAP solution was added on top of BNC and MBNC pellicles and allow to react for 1 min. Subsequently, 0.1 N of HCl was added per sample to stop the activation. The pellicles were washed with PBS and 200 ul of collagen was added on top per sample. The biocompatibility of the samples was tested via calcein/ethidium homodiamer-1 to mark healthy and apoptotic human smooth muscle cells (HSMCs) respectively.
MBNC characterization: XPS was employed to reveal the chemical composition both of MBNC and collagen-bioconjugated MBNC pellicles. Magnetic strength via VSM was used to measure the magnetic strength of the MBNC. SEM was used to examine the morphology of the MBNC.
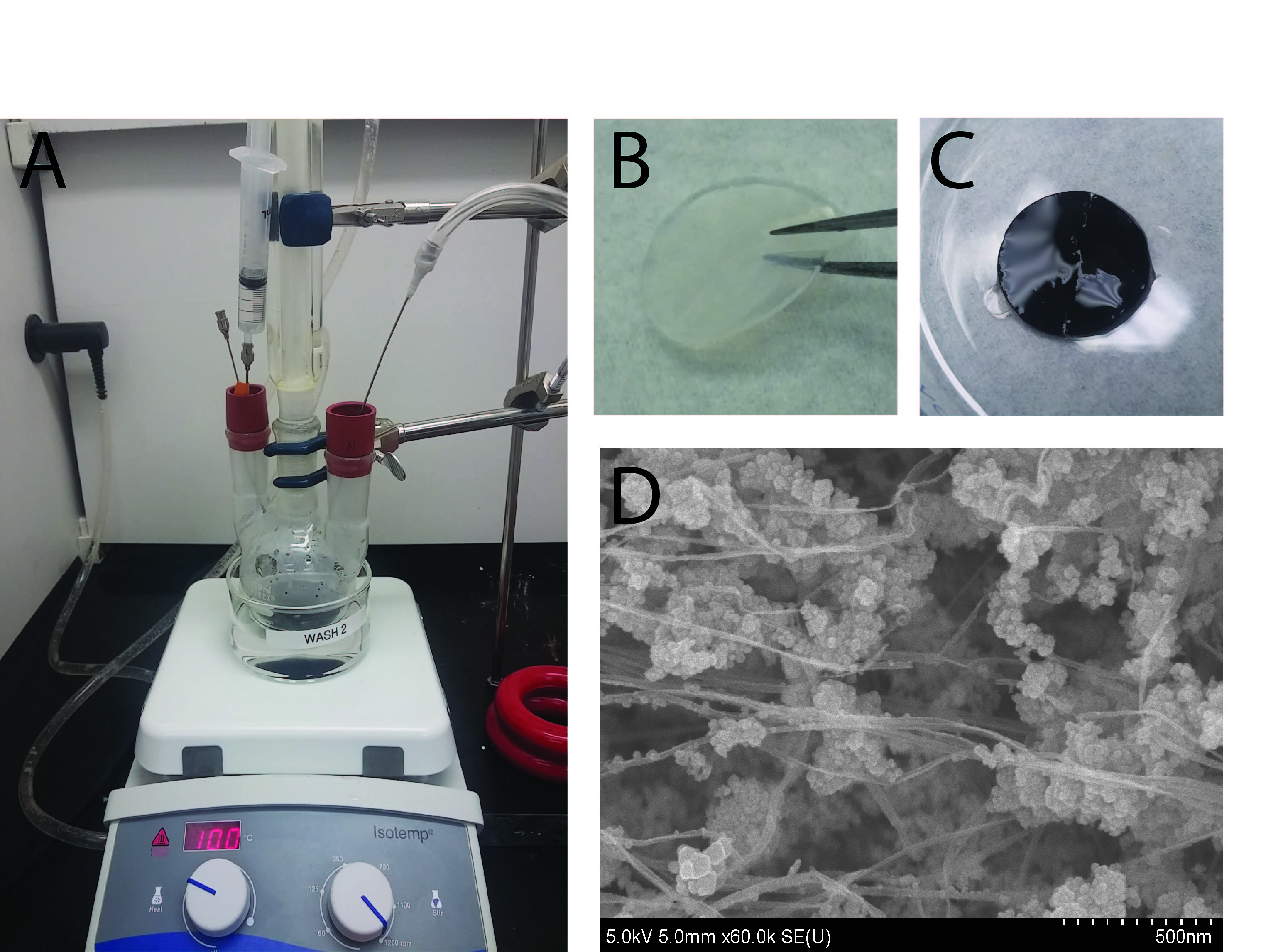
Results and Discussion: Fig. 1A shows the set-up used in the fabrication of MBNC. The BNC acquires a black color after incorporation of IONPs, which are dispersed along the BNC fibers or forming aggregates (Fig. 1B-D).
Compared with the XPS of BNC (Fig. 2), the spectrum of MBNC shows a significant new signal at a binding energy (BE) of 722 and 709 eV, which is the characteristic peak of Fe 2p1/2 and Fe 2p3/2. The XPS on collagen-conjugated MBNC resulted on the detection of nitrogen, which can be interpreted as an indirect measure of the protein content.
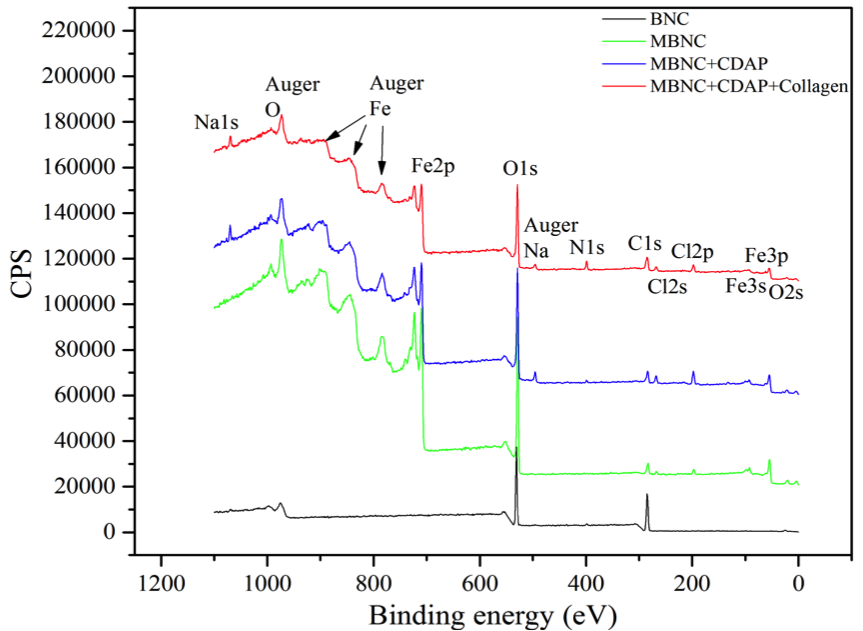
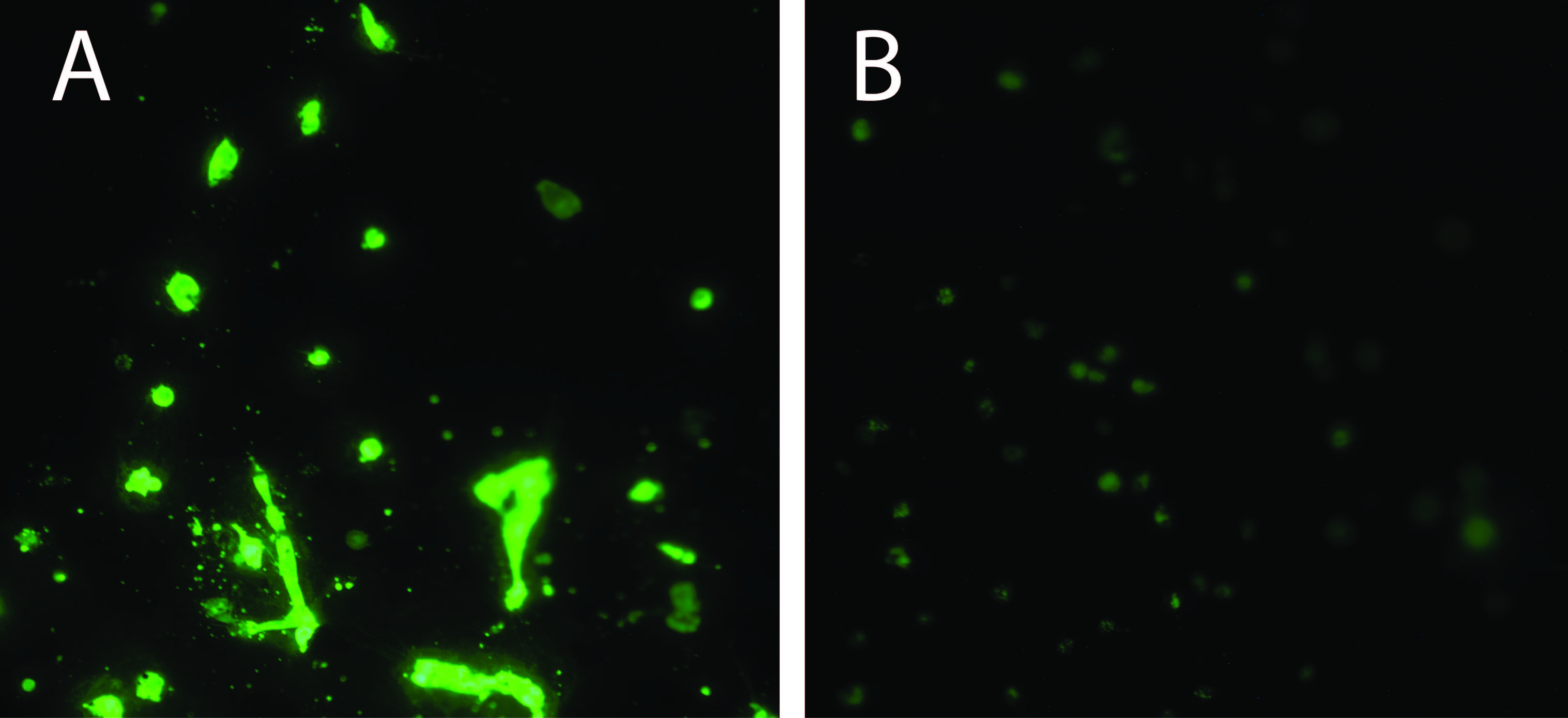
Fig. 3 shows the esterase activity measured via calcein (green) of HSMCs culture on MBNC for 24 hours. Both samples show a high HSMC viability and confluence, indicanting that the MBNC and collagen-bioconjugated MBNC did not have negative detrimental effects on HSMCs under these experimental conditions.
.
The VSM plot of MBNC provide evidence that IONPS are superparamagnetic at room temperature.
Conclusion: Our results show that we successfully incorporated Fe3O4 nanoparticles and cell-adhesion sites on the BNC. The VSM data showed that MBNC had a magnetic saturation of (0.054), which is above of the value considered to have satisfactory magnetic strength for tissue engineering purposes (0.049 emu/g).
This work was funded by Department of Defense under contract No. W81XWH-11-2-0067
References:
[1] de Barros Faria, M., Castro, R. N., Lundquist, J., Scrivano, E., Ceratto, R., Ferrario, A., & Lylyk, P. (2011). The role of the pipeline embolization device for the treatment of dissecting intracranial aneurysms. American Journal of Neuroradiology, 32(11), 2192-2195.
[2] Hsieh, Y. C., Yano, H., Nogi, M., & Eichhorn, S. J. (2008). An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose, 15(4), 507-513.
[3] Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Gray, D., & Dorris, A. (2011). Nanocelluloses: a new family of nature‐based materials. Angewandte Chemie International Edition, 50(24), 5438-5466
[4] Arias, S.L., Shetty, A., Senpan, A., Echeverry-Rendon, M., Allain, J.P. (2015) Fabrication of a functionalized magnetic bacterial nanocellulose with iron oxide nanoparticles. Journal of Visualized Experiments (Jove) (In-Press)
Keywords:
blood vessel,
nanoparticle,
Functionalization,
endothelialization
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
General Session Oral
Topic:
Biomaterials for cardiovascular applications, vascular grafts and embolic devices
Citation:
Arias
S,
Shetty
A,
Senpan
A,
Echeverry-Rendon
M and
Allain
J
(2016). Magnetic Bacterial Nanocellulose for neurovascular reconstruction in cerebral aneurysm treatment applications.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02134
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Sandra L Arias, University of Illinois at Urbana-Champaign, Department of Bioengineering, Urbana, IL, United States, ariassu2@illinois.edu