Recent developments in additive manufacturing have enabled the production of implantable metallic devices with highly mutable structures, including porous designs, and it is now possible to perform systematic studies of the efficacy of porous materials for supporting tissue in-growth. Studies of porous implants performed to date have largely focused on orthopedic applications. While a small number of studies have shown that highly porous devices, when implanted into subcutaneous tissue, facilitate fibrous tissue in-growth with significant attachment strength[1]-[7], the exact mechanism of soft tissue in-growth remains unclear. Further, no in-vivo analyses of transcutaneous porous implants, in which the implants penetrate through all dermal layers and into subcutaneous tissue, have been performed. Such studies are required in order to determine the potential for porous implants to promote wound healing in applications where contamination of the tissue-implant interface is unavoidable, such as percutaneous pin fixation of orthopedic fractures. We hypothesized that skin in-growth into a transcutaneous implant may form an effective biological seal, which would prevent bacteria from migrating into the subcutaneous and deeper tissues, causing infections. Reduction of infections could promote the development of healthy tissue in-growth in subcutaneous layers. The present pilot study was performed to determine the efficacy of additively manufactured, highly porous devices implanted in a transcutaneous fashion, and to qualitatively analyze the soft tissue in-growth capacity of such devices. A rabbit model was employed in the present study. Each of eight rabbits received four transcutaneous implants (two plastic, two titanium) for durations of one, two, three or seven weeks (two rabbits per time period). Implants harbored a rhombic dodecahedral porous network, or separately, a random highly porous foam network. Histology and histomorphometry were performed on implants from one rabbit at each post-surgical time period, including surrounding tissues, after surgical removal on the day of sacrifice. Implants were evaluated for responses consistent with inflammation and infection.
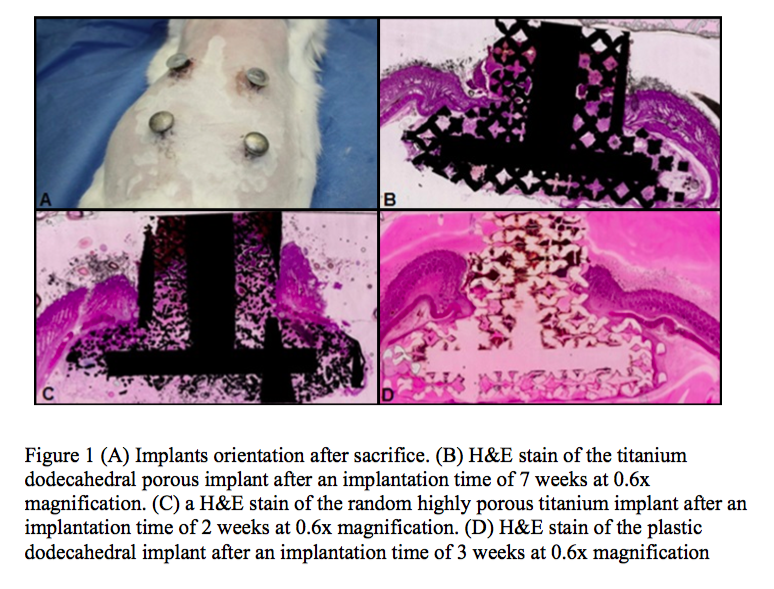
The percentage of in-growth of soft tissues (granulation tissue, subcutaneous tissue and dermis) based on surface area was evaluated. Transcutaneous, porous implants comprised of either titanium or plastic promoted soft tissue in-growth as early as one week post-surgery and remained uninfected at, or deep to, the biologic interface between the dermal layer and the implant for up to seven weeks post surgery.
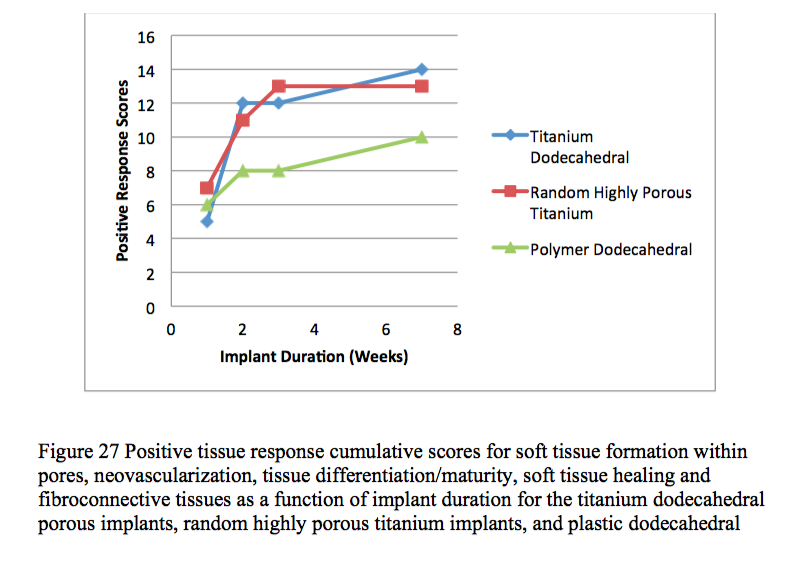
Histological and histomorphological analyses showed that skin, subcutaneous and granular tissue grew into the porous networks of both the titanium and plastic implants.
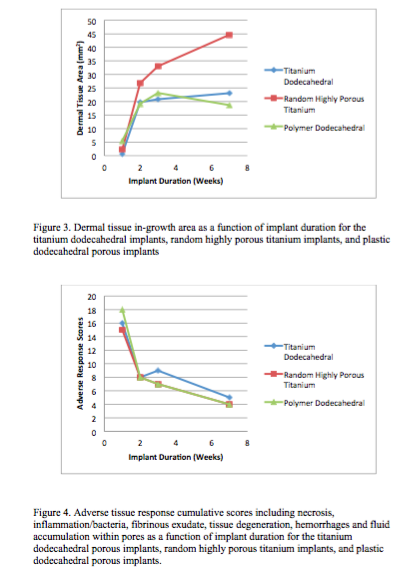
The present study demonstrated the efficacy of highly porous transcutaneous implants. Skin in-growth into porous transcutaneous implants formed an effective biological seal, preventing the establishment of infection-causing bacteria in tissues adjacent to the implants. Titanium implants with dodecahedral pore geometries performed better in several aspects than the plastic implants with dodecahedral pore geometries. Titanium implants with random highly porous foam geometry were more successful at promoting dermal tissue in-growth than titanium implants with uniform dodecahedral geometry.
The authors gratefully acknowledge the University of Maine Institute for Molecular Biophysics for funding, AccelLab (Montreal, Canada) for performing histology and histomorphology analyses, Alexander French of the University of Maine Advanced Manufacturing Center for CAD design work, and GROWit, and Stryker Corporation for manufacturing the implants used in the present study.
References:
[1] Basalah, A.; Shanjani, Y.; Esmaeili, S.; Toyserkani, E. Characterizations of Additive Manufactured Porous Titanium Implants. J. Biomed. Mater. Res. B. Appl. Biomater. 2012, 100 (7), 1970–1979.
[2] El-Hajje, A.; Kolos, E.; Wang, J.; Maleksaeedi, S.; He, Z.; Wiria, F.; Choong, C.; Ruys, A. Physical and Mechanical Characterisation of 3D-Printed Porous Titanium for Biomedical Applications. J. Mater. Sci. Mater. Med. 2014, 25 (11), 2471–2480.
[3] Xiong, Y.; Qian, C.; Sun, J. Fabrication of Porous Titanium Implants by Three-Dimensional Printing and Sintering at Different Temperatures. Dent. Mater. J. 2012, 31 (5), 815–820.
[4] Bobyn, J. D.; Wilson, G. J.; MacGregor, D. C.; Pilliar, R. M.; Weatherly, G. C. Effect of Pore Size on the Peel Strength of Attachment of Fibrous Tissue to Porous-Surfaced Implants. J. Biomed. Mater. Res. 1982, 16 (5), 571–584.
[5] Hacking, S. A.; Bobyn, J. D.; Toh, K.; Tanzer, M.; Krygier, J. J. Fibrous Tissue Ingrowth and Attachment to Porous Tantalum. J. Biomed. Mater. Res. 2000, 52 (4), 631–638.
[6] LaBerge, M.; Bobyn, J. D.; Rivard, C. H.; Drouin, G.; Duval, P. Study of Soft Tissue Ingrowth into Canine Porous Coated Femoral Implants Designed for Osteosarcomas Management. J. Biomed. Mater. Res. 1990, 24 (7), 959–971.
[7] Abdelaal, O. A.; Darwish, S. M. Fabrication of Tissue Engineering Scaffolds Using Rapid Prototyping Techniques. World Acad. Sci. Eng. Technol. Int. Sci. Index 2011, 59, 1325–1333.