Introduction: Mg alloys are increasingly attractive candidates for hard and soft tissue implants due to their long term functionality, superior mechanical properties over polymers and their ability to be absorbed and excreted by the body, thus removing the need for surgical implant removal. However, the corrosion processes of Mg alloys may lead to adverse cellular responses. Thus specific biocompatibility evaluation is required. A degradable paediatric tracheal stent should remain functional for 6-18 months to allow the completion of biological remodelling [1]. During this time its surface topography will alter. Furthermore, the toxicity of a metallic implant are influenced by its composition, component elements and surface roughness [2]. However, studies on the biocompatibility of AZ31 Mg alloys after long term in vitro degradation have not been reported. The aim of this study was to assess the influence of up to 6 months in vitro corrosion on the surface roughness (topography) and cell viability of AZ31 Mg alloys.
Materials and Methods: Degradation test: AZ31 Magnesium alloy specimens were immersed in Phosphate Buffered Saline (PBS) at a SA:V of 0.4ml/mm2 and incubated at 37oC for 1, 3, or 6 months. The degradation media was renewed every 2-5 days, with specimens rinsed in water and cleaned with ethanol prior to re-emersion.
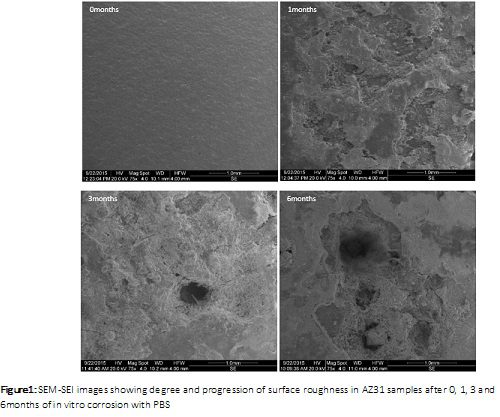
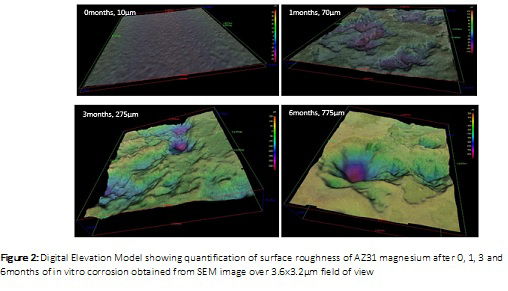
Fractography: Samples were imaged using a Zeiss Sigma Scanning Electron Microscope (SEM) operating at 20kV. Secondary electron images (SEI) and Digital Elevation Model (DEM) were used to analyse the surface topography.
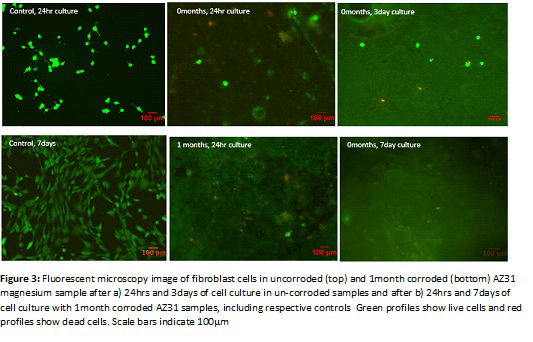
Cell culture: Fibroblast cells (50x104 cells/ml) were seeded onto the surface of AZ31 specimens (10x10x2mm) post in vitro corrosion. Direct contact method and a live/dead cytotoxicity assay were used to assess the biocompatibilty after 1, 3 and 7 days culture. Tissue culture plastic was used as controls. After staining, cells were viewed using an Olympus EX51 fluorescence microscope.
Results: SEM fractography of AZ31 revealed increased surface roughness with increasing degradation. Further, corrosion induced pits, grooves and surface defects become more widespread and deeper with increasing degradation time.
The maximum depth of the degradation pits was determined to be 10µm, 70µm, 275µm, 775µm in AZ31 samples corroded for 0, 1, 3, and 6 months respectively. Cell viability was confirmed in degraded and non degraded AZ31 magnesium samples after days of in vitro culture with fibroblasts despite the formation of hydrogen bubbles (corrosion by-product) during the cell culture.
Live cells adopted a more contracted morphology when attached to rough corroded surfaces compared with smooth surfaces of un-corroded Mg AZ31. Fibroblast cells varied in size following culture from approximately 20-300µm, a size distribution that has been highlighted in literature [3].
Conclusions: AZ31 alloy was cytocompatible with fibroblast cells after long term in vitro corrosion and can be used in biodegradable paediatric tracheal stents.
Action Medical Research
References:
[1] Hermawan, H., Dubé, D., Mantovani, D., Review. Developments in metallic biodegradable stents Acta Biomaterialia 6 (2010) 1693–1697
[2] Hanawa T. Evaluation techniques of metallic biomaterials in vitro. Sci Technol
Adv Materials 2002;3:289–95.
[3] Jozsa, L., and Kannus, P., Human Tendons: Anatomy, Physiology and Pathology. Human Kinetics Champaign, IL., 1997: p. 1-574