Bone extracellular matrix (ECM) consists in self-assembled nanostructure of proteins (mainly type I collagen) and poor crystalline hydroxyapatite nanocrystals (HA) that surrounds and affects mesenchymal stem cell adhesion, proliferation and differentiation through physico-chemical processes[1]. In attempts to orchestrate in vivo bone environment, we propose biomineralization of a synthetic collagen peptide (SCP, manufactured as CellnestTM by FUJIFILM Europe B.V.). Furthermore, inspired by non-stoichiometric composition of bone apatite, magnesium (Mg) is introduced during mineralization process. Mg controls HA crystallization process as well as can stimulate the osteoblast proliferation[2] and inhibit bacterial adhesion[3]. Variation on morphological and chemical features of HA related to the effect of SCP and Mg during mineralization affect surface nanotopography and consequently micro- and macroscopic interfacial scaffold properties with great relevance on cell interaction.
Mineralization of synthetic collagen like-peptide (SCP) was carried out by neutralization process[4] in the absence (SCP-HA) and in the presence of magnesium (SCP-MgHA). The effect of SCP and Mg in HA crystallization was evaluated through the morphological characterization by X-Ray powder diffraction (patterns analyzed by Debye Function Analysis[5]) and transmission electron microscopy (TEM). Chemical analysis was performed by Raman spectroscopy, inductively coupled plasma optical emission spectrometry (ICP-OES) and thermogravimetric analysis. Looking ahead, mineralized solution was directly freeze-dried for the development of porous 3D scaffolds. After that, scaffolds were chemical crosslinked to ensure their stability for long-term biomedical applications. Scaffold surface topography was assessed by High-Resolution Atomic Force Microscopy (HR-AFM). The biological response of MC3T3-E1 cell line to scaffolds was assessed, too.
Poor crystalline carbonated HA with similar features to that of bone apatites was synthesized. The amorphous calcium phosphate phase (ACP) was present in all conditions being the main phase in SCP-MgHA mineralization experiments which indicated the synergistic effect of SCP and Mg in stabilization of amorphous phase. Moreover, HR-AFM images of scaffold surface showed that mineralization under this condition (SCP-MgHA) lead to homogeneous mineral distribution while non-covered zones together with isolated HA nanocrystals and/or HA aggregates appeared when mineralization is carried out in the absence of Mg (SCP-HA). MC3T3-E1 cells spread inside the material and exhibited their characteristic morphology which could indicate the osteoconductive effects of designed scaffolds.
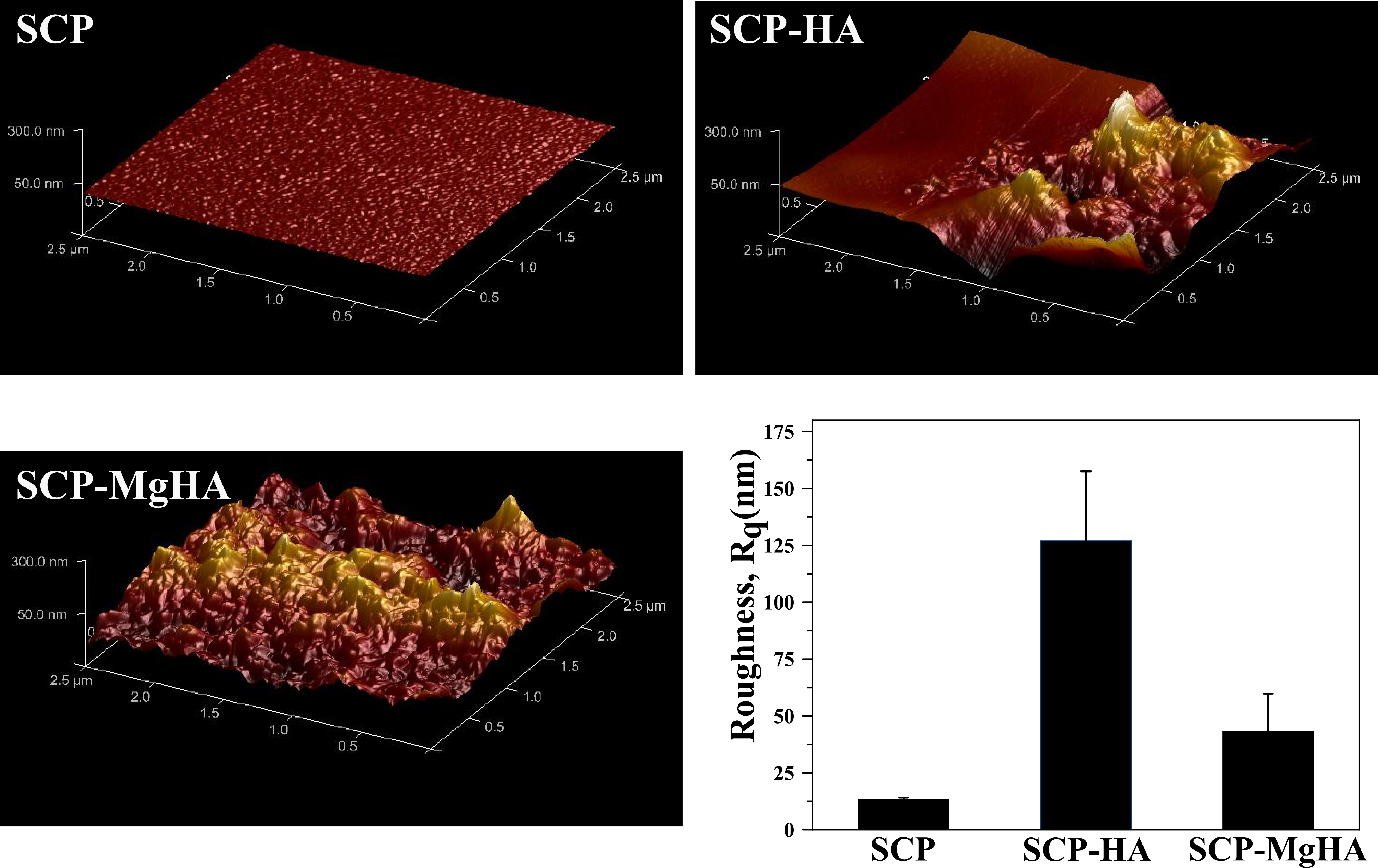
Figure 1. 3D HR-AFM images of non-mineralized and mineralized scaffold surface as well as surface roughness in terms of root mean square (RMS) roughness (Rq) for scan area 2.5µm x 2.5 µm.
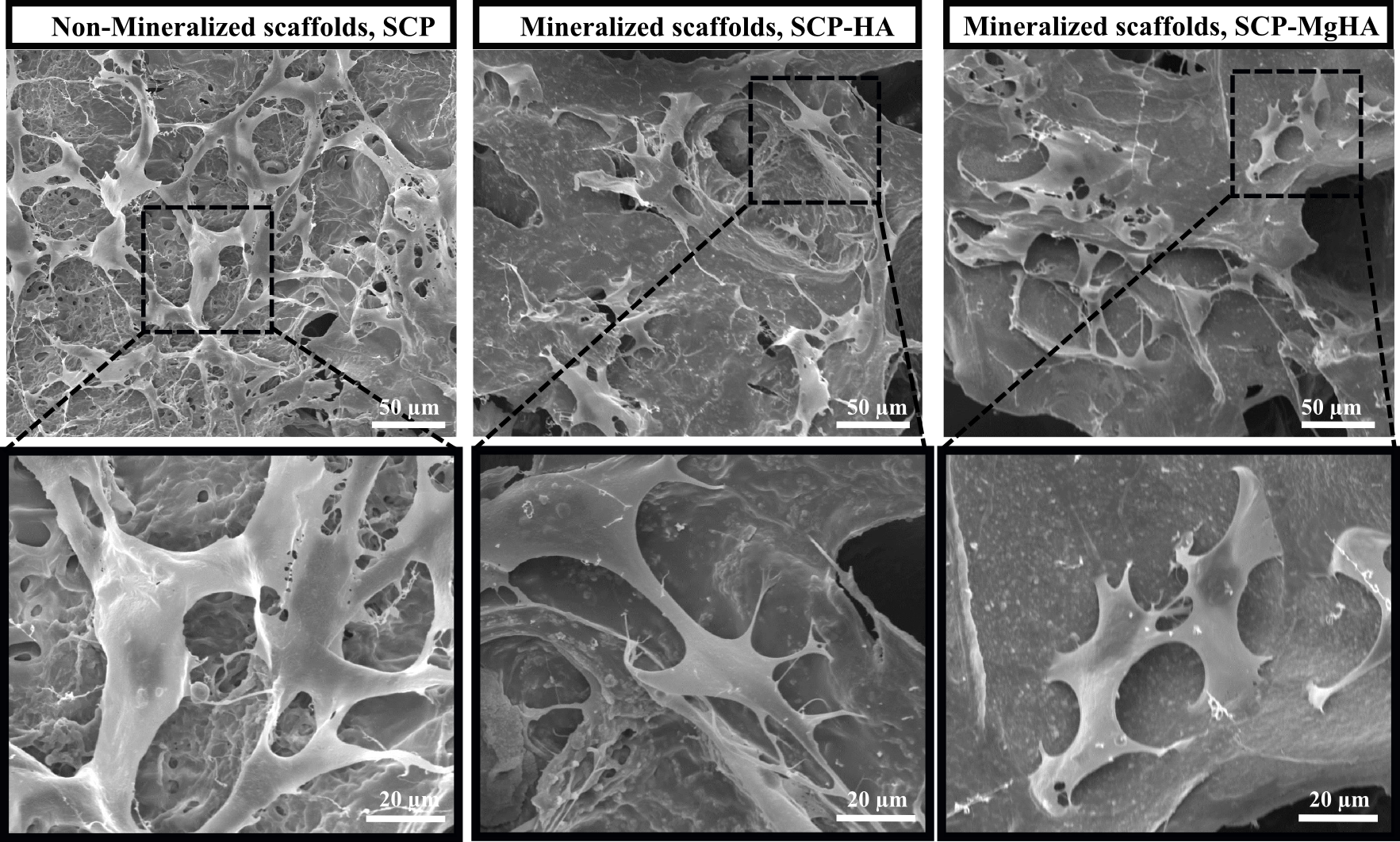
Figure 2. SEM micrographs of MC3T3-E1 cells attached to non-mineralized and mineralized scaffolds after 7 days of culture.
Herein, we propose biomineralization as a versatile route for the development of biomaterials for bone tissue engineering from nano- to macro-scopic scale. The introduction of foreign ions during mineralization allows us to modify physico-chemical properties that cue cell behavior. In our studies, synergistic effect of SCP and Mg on mineralization provided a homogeneous amorphous mineral distribution triggering to a more resorbable biomaterial. In vitro studies showed that cells attached, proliferated and colonized the scaffolds after 7 days.
The research leading to these results has received funding from the European Union Seventh Framework Programme FP7-PEOPLE-2013-ITN under grant agreement n 607051
References:
[1] R. Ayala, C. Zhang, D. Yang, Y. Hwang, A. Aung, S. S. Shroff, F. T. Arce, R. Lal, G. Arya, S. Varghese, Biomaterials 2011, 32, 3700-3711.
[2] J. Chou, S. Valenzuela, J. Santos, D. Bishop, B. Milthorpe, D. Green, M. Otsuka, B. Ben‐Nissan, Journal of tissue engineering and regenerative medicine 2014, 8, 771-778.
[3] R. Ma, Y.-x. Lai, L. Li, H.-l. Tan, J.-l. Wang, Y. Li, T.-t. Tang, L. Qin, Scientific reports 2015, 5.
[4] A. Tampieri, G. Celotti, E. Landi, M. Sandri, N. Roveri, G. Falini, Journal of Biomedical Materials Research Part A 2003, 67, 618-625.
[5] J. M. Delgado-López, R. Frison, A. Cervellino, J. Gómez-Morales, A. Guagliardi, N. Masciocchi, Adv. Funct. Mater. 2014, 24, 1090-1099.