Introduction: Phosphate based glasses (PBGs) are an ideal material to act as “carriers” of therapeutic ions such as strontium (Sr), since their degradation rate can be tailored to range over orders of magnitude [1] by altering the glass composition. Sr substitution is of particular interest for orthopaedic applications due to the dual effect of Sr on bone metabolism: osteoblast activation and osteoclast inhibition[2]. This study aims to investigate the effect of the substitution of CaO with SrO in a PBG system with a fixed glass former content of 40mol%. By investigating the glass structure at an atomic level the inter-relationship between glass composition, structure and durability can be understood, enabling optimisation of an ideal therapeutic glass with controlled release rates.
Materials and Methods: SrO was substituted for CaO in a series of PBGs (mol%(P2O5)40 (CaO)16-x (MgO)24 (Na2O)20 (SrO)x where x= 0, 4, 8, 12 and 16, denoted P40, Sr4, Sr8, Sr12 & Sr16 respectively). All glass samples were fabricated via a melt quench route involving drying of precursors at 350ºC for 30min, followed by melting at 1150ºC for 1.5h. Structural characterisation was carried out using 31P NMR (spectra obtained at the EPSRC UK National Solid-state NMR Service, Durham) and neutron diffraction (ND) studies (GEM diffractometer at ISIS spallation neutron source, Rutherford Appleton Laboratory, UK). The ND results were interpreted using fitting and simulation techniques. Dissolution studies were conducted in distilled water at 37ºC for 28d.
Results and Discussion: The ND study revealed that CaO substitution with SrO had little effect on the well-ordered, short range structure of the glasses. This is thought to be due to the similarities in charge and field strength of Ca2+ and Sr2+ ions. The coordination numbers (CN) derived from ND showed that all P atoms are 4-coordinated (Table 1) which is consistent with previous studies[3]. The modifier environments for each glass composition also did not vary considerably as shown in Figure 1A & B. Solid state 31P NMR indicated little change in Qi species for all compositions except for Sr16. These glasses showed an approximate 1:1 ratio of Q1:Q2 species, suggesting a network connectivity (number of bridging oxygens (BO) per network forming tetrahedral) of around 1.5. From these results it can be assumed the glass network consists of an average chain length of 4 tetrahedral units. The larger increase in Q1 species for Sr16 indicated that Q1 units may also be present as dimers i.e. pyrophosphates. Dissolution studies showed that Sr free glasses degraded at a faster rate than those with Sr, which contradicted previous studies [4][5]. In addition, no significant difference was observed between the Sr containing glasses (p>0.05) suggesting it may be possible to deliver varying concentrations of Sr without affecting the rate of the glass degradation.
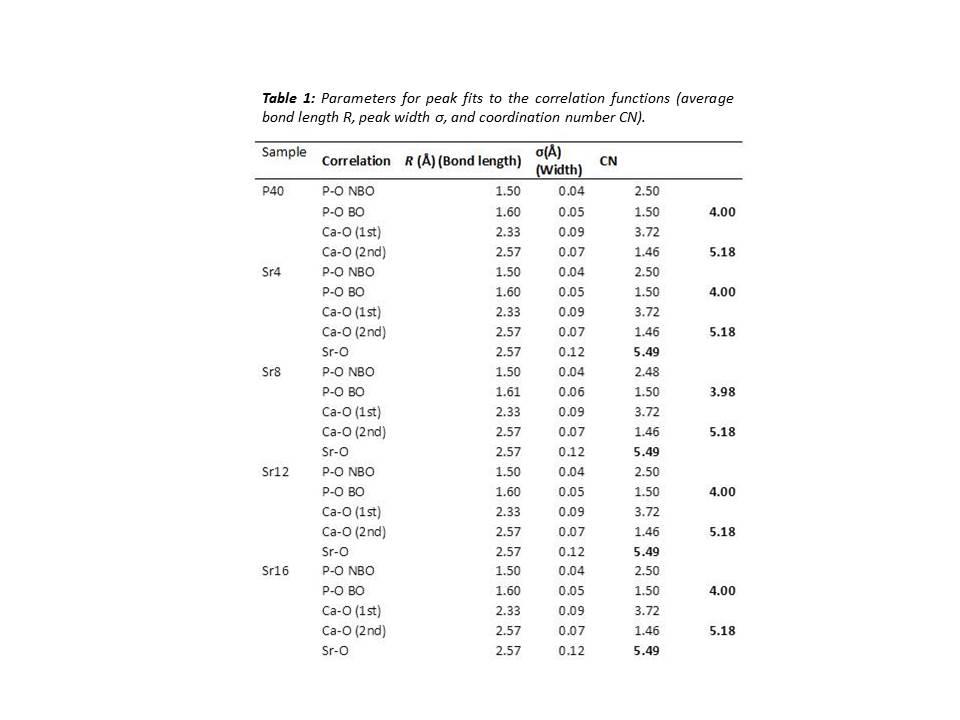
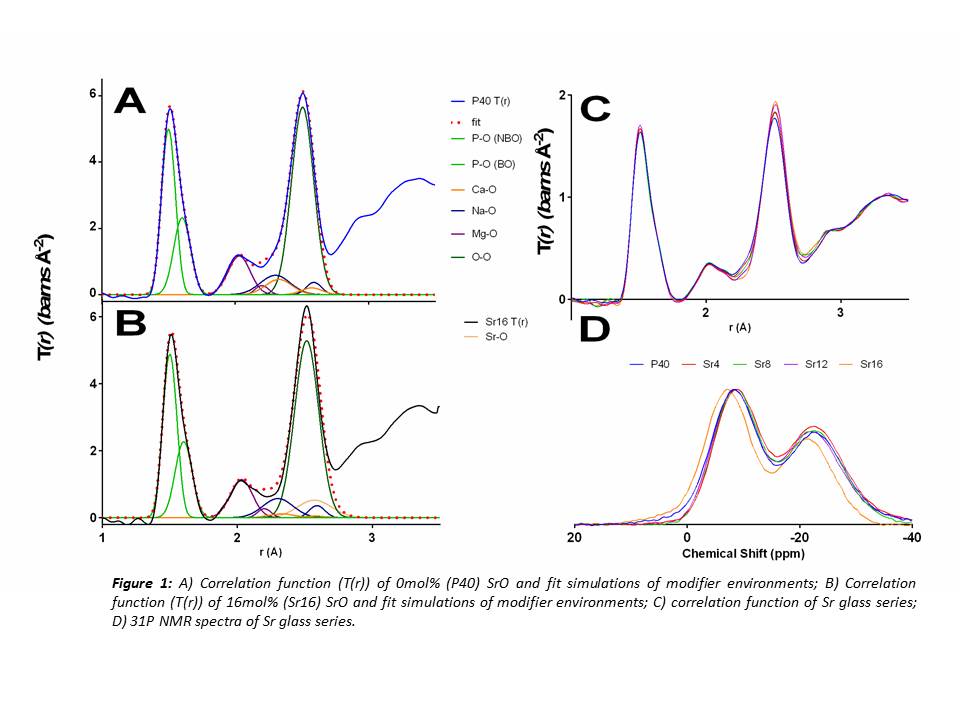
Conclusion: Replacing CaO with SrO exerted little effect on the overall glass structure. A consistent Qi distribution and network connectivity was observed along with little change in modifier environments (CN and percentage of BO). The dissolution studies showed that the Sr content had an insignificant effect on degradation rate, hence controlled release of a suited amount of Sr may be delivered posing these glasses beneficial for therapeutic use in bone related diseases such as osteoporosis.
Funding body: Science and Technology Facilities Council (STFC ref. no. ST/L502583/1); EPSRC UK National Solid-state NMR Service at Durham
References:
[1] Bunker BC, Arnold GW, Wilder JA. Phosphate-Glass Dissolution in Aqueous-Solutions. J Non-Cryst Solids. 1984;64:291-316.
[2] Boivin G, Doublier A, Farlay D. Strontium ranelate - a promising therapeutic principle in osteoporosis. J Trace Elem Med Bio. 2012;26:153-6.
[3] Pickup DM, Ahmed I, Guerry P, Knowles JC, Smith ME, Newport RJ. The structure of phosphate glass biomaterials from neutron diffraction and P-31 nuclear magnetic resonance data. J Phys-Condens Mat. 2007;19.
[4] Abou Neel EA, Chrzanowski W, Pickup DM, O'Dell LA, Mordan NJ, Newport RJ, et al. Structure and properties of strontium-doped phosphate-based glasses. J R Soc Interface. 2009;6:435-46.
[5] Al Qaysi M, Walters NJ, Foroutan F, Owens GJ, Kim HW, Shah R, et al. Strontium- and calcium-containing, titanium-stabilised phosphate-based glasses with prolonged degradation for orthopaedic tissue engineering. J Biomater Appl. 2015.