Introduction: Gene therapy has become alternative therapeutic approach to treat genetic diseases such as cancer[1]. Nonviral vectors have gained tremendous attraction over the viral vector due to its low toxicity, non-immunogenicity and low production cost[2]-[4]. Recently, rare earth metal oxide especially cerium oxide nanoparticle (CNP) has widely been used in biomedical fields such as drug delivery, bio-imaging, nanomedicine and tissue engineering application because of its inherent antioxidant activity, free radical scavenging activity and radioprotection to normal cell[5],[6]. It is also reported that CNP increased the ROS (reactive oxygen species) in cancer cell[7]. Therefore, we hypothesized that therapeutic gene (like siRNA, p53 etc.) delivery by means of CNP may give the dual therapeutic effect in conjugation with increased ROS level in cancer cell for the treatment of cancer. Here we report the functionalization of CNP by low molecular weight polyethyleneimine (LPEI, 2 kDa) followed by conjugation of folic acid (FA) for targeted gene delivery.
Methods: LPEI was conjugated with CNP (LPEI-conj-CNP) through carboxylation followed by amidation reaction. Then, FA was reacted with LPEI-conj-CNP by carbodiimide to get FA-LPEI-conj- CNP. CNP/pDNA (enhanced green fluorescent protein, EGFP encoding pDNA) complexes were prepared at various weight ratios and characterized by agarose gel electrophoresis assay and dynamic light scattering (DLS). In vitro transfection efficiency, toxicity, ROS and underlying mechanistic pathway were also evaluated in HeLa cell.
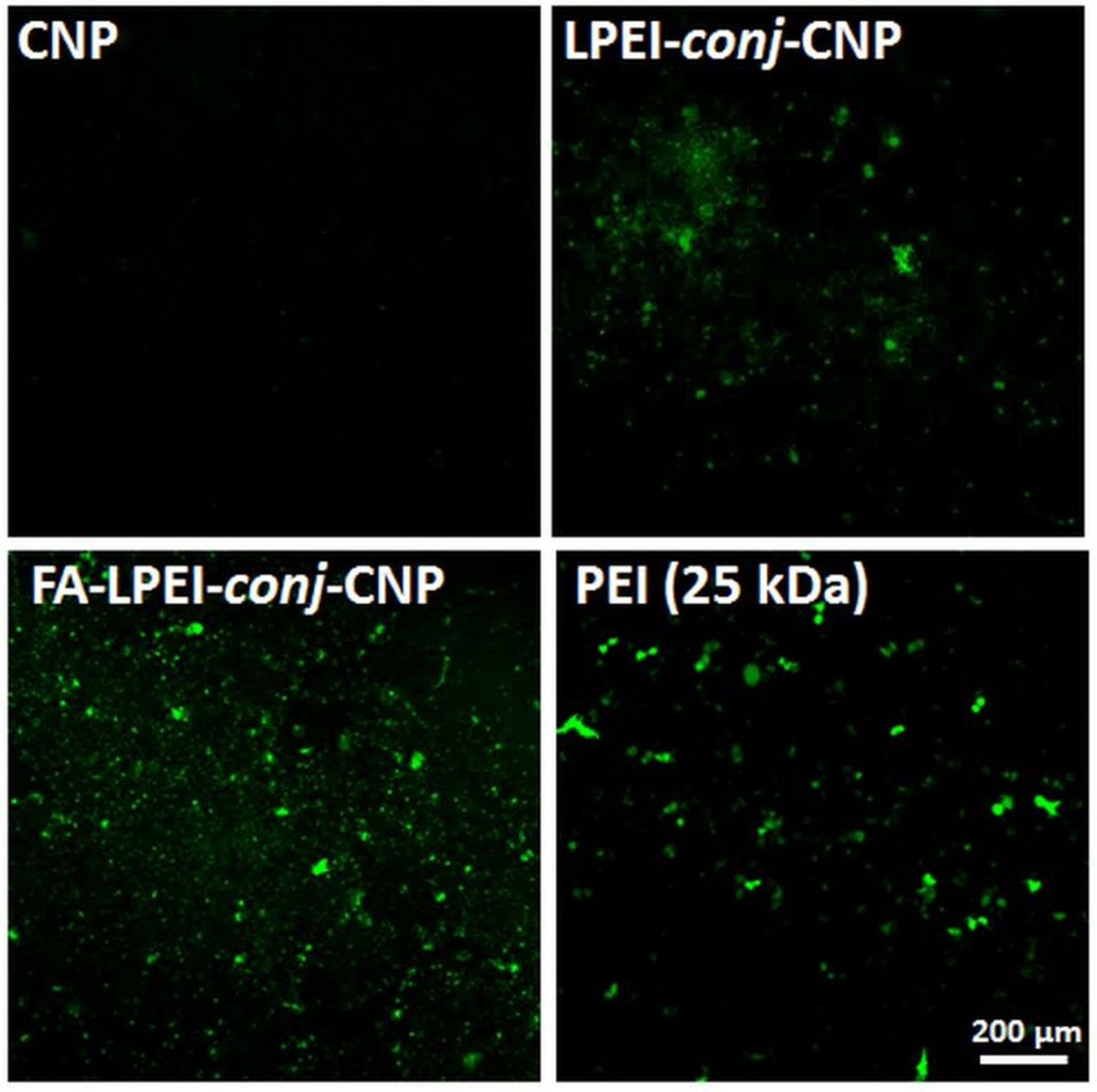
Results: Poor water dispersibility of CNP at physiological pH is a major drawback for biological field especially gene/ drug delivery application. CNP forms stable dispersion in acidic pH only. Despite the positive zeta potential of CNP, it did not bind any DNA at any weight ratio due to poor dispersibility. But, the DNA complexation capability of CNP significantly increased after conjugation of LPEI and LPEI-conj-CNP complexed all pDNA at a very low weight ratio of 0.5:1. Due to presence of lots of primary amine groups in LPEI, the water dispersibility of CNP increased drastically and resulted better DNA complexation through the cationic primary amine groups. The transfection efficiency of CNP increased after conjugation of LPEI but the efficiency increased significantly after FA conjugation with LPEI-conj-CNP. FA-LPEI-conj-CNP/pDNA complex at weight ratio of 15:1 showed highest transfection efficiency which also surpassed the efficiency offered by PEI (25 kDa) (Figure 1). It was also observed that FA-LPEI-conj-CNP internalized into cell via folate receptor as well as caveolae mediated pathway and resulted enhanced transfection efficiency. While LPEI-conj-CNP followed clathrin mediated pathway followed by lysosomal compartment entrapment and as a result showed low transfection efficiency.
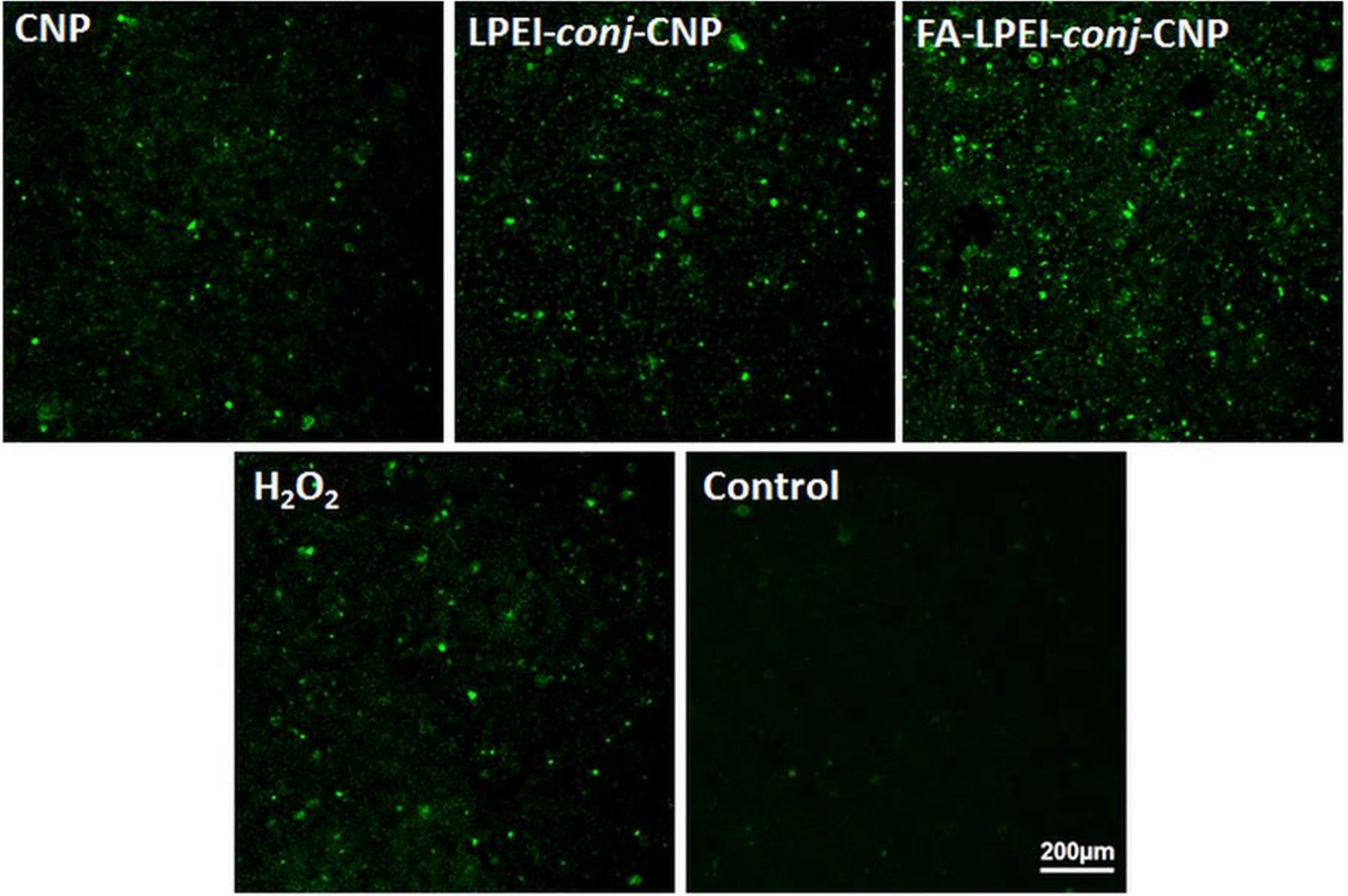
It was found that green fluorescence (fluorescent dichlorofluorescein, DCF obtained through oxidation of nonfluorescent dichlorofluorescein diacetate, DCFA by ROS) increased drastically by FA-LPEI-conj-CNP compared to all CNPs and H2O2 treated cell indicating the increase in ROS (Figure 2).
Conclusion: The transfection efficiency as well as ROS of CNP increased after functionalization of CNP especially after FA conjugation. Therefore, FA-LPEI-conj-CNP may be useful candidate in nonviral gene therapy for cancer treatment by using its dual property including gene delivery and ROS enhancement.
This work was financially supported by Start-up funds from University of Pittsburgh School of Pharmacy
References:
[1] Amer, M. H. Gene therapy for cancer: present status and future perspective. Molecular and Cellular Therapies 2014, 2, 27.
[2] Kundu, P. P.; Sarkar, K. Natural Polymeric Vectors in Gene Therapy. In Biopolymers, John Wiley & Sons, Inc.: 2011; pp 575-603.
[3] Yin, H.; Kanasty, R. L.; Eltoukhy, A. A.; Vegas, A. J.; Dorkin, J. R.; Anderson, D. G. Non-viral vectors for gene-based therapy. Nat Rev Genet 2014, 15, 541-555.
[4] Lundstrom, K.; Boulikas, T. Viral and Non-viral Vectors in Gene Therapy: Technology Development and Clinical Trials. Technology in Cancer Research & Treatment 2003, 2, 471-485.
[5] Xu, C.; Qu, X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater 2014, 6, e90.
[6] Walkey, C.; Das, S.; Seal, S.; Erlichman, J.; Heckman, K.; Ghibelli, L.; Traversa, E.; McGinnis, J. F.; Self, W. T. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environmental Science: Nano 2015, 2, 33-53.
[7] Wason, M. S.; Zhao, J. Cerium oxide nanoparticles: potential applications for cancer and other diseases. American Journal of Translational Research 2013, 5, 126-131.