Newborn analgesia mediated by oxytocin during delivery
- 1 INMED/INSERM U901, Université de la Méditerranée, Marseille, France
- 2 Department of Audiology, Université Claude Bernard Lyon I, Lyon, France
- 3 Department of Neurobiology, A.I. Virtanen Institute, University of Eastern Finland, Kuopio, Finland
- 4 Kazan Institute of Biochemistry and Biophysics, Kazan Scientific Center of Russian Academy of Sciences, Kazan, Russia
- 5 Spinal laboratory SISSA - IMFR, Udine, Italy
The mechanisms controlling pain in newborns during delivery are poorly understood. We explored the hypothesis that oxytocin, an essential hormone for labor and a powerful neuromodulator, exerts analgesic actions on newborns during delivery. Using a thermal tail-flick assay, we report that pain sensitivity is two-fold lower in rat pups immediately after birth than 2 days later. Oxytocin receptor antagonists strongly enhanced pain sensitivity in newborn, but not in 2-day-old rats, whereas oxytocin reduced pain at both ages suggesting an endogenous analgesia by oxytocin during delivery. Similar analgesic effects of oxytocin, measured as attenuation of pain-vocalization induced by electrical whisker pad stimulation, were also observed in decerebrated newborns. Oxytocin reduced GABA-evoked calcium responses and depolarizing GABA driving force in isolated neonatal trigeminal neurons suggesting that oxytocin effects are mediated by alterations of intracellular chloride. Unlike GABA signaling, oxytocin did not affect responses mediated by P2X3 and TRPV1 receptors. In keeping with a GABAergic mechanism, reduction of intracellular chloride by the diuretic NKCC1 chloride co-transporter antagonist bumetanide mimicked the analgesic actions of oxytocin and its effects on GABA responses in nociceptive neurons. Therefore, endogenous oxytocin exerts an analgesic action in newborn pups that involves a reduction of the depolarizing action of GABA on nociceptive neurons. Therefore, the same hormone that triggers delivery also acts as a natural pain killer revealing a novel facet of the protective actions of oxytocin in the fetus at birth.
Introduction
Delivery is stressful and potentially painful event for the newborn (Lagercrantz and Bistoletti, 1977; Bistoletti et al., 1983; Hagnevik et al., 1984; Lagercrantz and Slotkin, 1986; Fitzgerald, 2005). Sources of pain during delivery can be natural, such as mechanical compression of the fetus (Derek, 1999), and iatrogenic, such as forceps extraction, blood samples – including fetal scalp blood samples – and injections. Clinical studies indicate that painful experiences in neonates may disrupt the adaptation of newborn infants to their postnatal environment and in the long term, lead to psychological sequelae (Anand and Hickey, 1987). In mice, early exposure to noxious or stressful stimuli alters pain sensitivity and behavior in adult life, possibly by altering the stress-axis and antinociceptive circuitry (Sternberg et al., 2005; Laprairie and Murphy, 2009). Therefore, the problem of pain and its treatment in the newborn is of clinical importance (Anand, 2001; Slater et al., 2010); however, the mechanisms involved in pain regulation at birth are poorly understood.
Recently, comparison of the pain responses in human neonates born with vaginal delivery or elective cesarean section revealed diminished physiological, behavioral and vocalization responses to painful stimuli following vaginal delivery when compared to C-sections, suggesting that antinociceptive mechanisms are activated and last for few hours during and after normal delivery (Bergqvist et al., 2008). The mechanisms underlying this transient newborn analgesia at present remain unknown. Here, we explored an involvement of oxytocin in newborn analgesia. Oxytocin, who’s levels sharply increase during vaginal delivery, is not only involved in labor but also exerts multiple actions in the nervous system (Argiolas and Gessa, 1991; Gimpl and Fahrenholz, 2001; Raggenbass, 2001; Russell et al., 2003; Tomizawa et al., 2003; Huber et al., 2005; Kosfeld et al., 2005; Theodosis et al., 2006). In adult rats, oxytocin exerts an analgesic action (Arletti et al., 1993; Lundeberg et al., 1993, 1994; Agren et al., 1995; Petersson et al., 2001; Yu et al., 2003; Condés-Lara et al., 2006; Miranda-Cardenas et al., 2006). The analgesic effects of oxytocin in adults is mediated by GABAergic inhibition of the nociceptive inputs to the dorsal horn of the spinal cord (Condés-Lara et al., 2009). Nociception is strongly regulated not only by amount of the GABA(A) receptor mediated anionic conductance, but also by its reversal potential (EGABA), as depolarizing shifts in EGABA in the nociceptive and dorsal horn neurons are associated with elevated pain (De Koninck, 2007; Price et al., 2009). Pain is alleviated by pharmacological blockade or genetic knock out of NKCC1 chloride co-transporter, which is the primary cause for elevated chloride and depolarizing action of GABA in the nociceptive neurons (Delpire and Mount, 2002; Granados-Soto et al., 2005; Pieraut et al., 2007; Rocha-González et al., 2008). In the immature cortical neurons, oxytocin and NKCC1 blockers produce similar negative shift in EGABA (Tyzio et al., 2006; Khazipov et al., 2008). We now report that oxytocin causes pain relief in the newborn at birth, via reduction in depolarizing action of GABA on the nociceptive neurons.
Materials and Methods
Animals
All animal use protocols conformed to the INSERM guidelines and the Italian act Decreto Legislativo 27/1/92 n. 116 implementing the European Community directives n. 86/609 and 93/88 on the use of laboratory animals. Pregnant and maternal Wistar rats were housed with a 12-h light–dark cycle, at 24 ± 1°C, and food and water ad libitum. Pregnant rats were monitored hourly on the day of expected delivery. During delivery, which lasts in the rat from a 30 min to 1 h, newborn pups were numbered, weighted and stored in a warmed box prior to the pain assays. Delay from delivery to the onset of pain assays varied from 30 min to 1 h. Pups born first were assayed first. Together with the time for the pain tests and drug injections (tests for each drug were performed 30 min after injection) all the measurements were obtained within 2 h after birth.
Quantification of the Nociceptive Response
Tail immersion
The pup was held in a box with a hole allowing the tail to protrude from it. The inner surface of the box was covered with an aluminum sheet forming an electrical contact with the rat body. The electrical circuit via the sheet, body, tail, and water bath was powered by a 1.5-V battery and its connection and disconnection could be easily detected upon the tail immersion and withdrawal from the water, respectively. Electrical signals were digitized at 1 kHz using a Digidata 1200 and recorded to a computer. The distal tip of the tail was lowered into the water bath (50°C). Latency to withdrawal was recorded as the “pain” parameter, with a 15-s maximum allowable threshold. After three habituation tests, the latency to withdrawal was determined from the average of three consecutive measurements.
Vocalization
Under isoflurane (1.5%) anesthesia, rat pups were implanted with bipolar electrodes into the whisker pad and decerebrated at the upper pons level. After 10 min (P0) or 1 h (P1–2 pups) of recovery, the pups were wrapped by cotton and placed on a thermal blanket (38°C). The whisker pad was stimulated by electrical pulse trains (1 ms pulse duration, 5–25 V amplitude, 50 Hz, 1 min inter-train interval). It has been shown that pain vocalization includes A-delta and C-fibers – triggered audible “peeps” (with the most prominent peak at 4–5 kHz) and “chatters” (peak at 2.5 kHz) followed by ultrasonic vocalizations patterned by respiration cycles (Jourdan et al., 1995, 1997). In the present study, audible component of the vocalization response was recorded by microphone, digitized at 10 kHz using Digidata 1440 interface (Axon Instruments) and analyzed offline using Matlab (MathWorks, Natick, MA, USA). To quantify the vocalization response, we calculated scalar integral (Σ) as following: (i) raw sonogram was converted to scalar sonogram by inverting all negative values to positive values; (ii) scalar sonogram was corrected for the baseline activity level by subtraction of the mean scalar sonogram value calculated during 1 min before stimulation; (iii) scalar sonogram integral was calculated as cumulative, corrected for the baseline, scalar sonogram during 5 s after stimulation.
Calcium imaging
Trigeminal sensory neurons were obtained from P0 rats. Animals were anesthetized and decapitated. Trigeminal ganglia were excised and enzymatically dissociated in F12 medium containing 0.25 mg/ml trypsin, 1 mg/ml collagenase, and 0.2 mg/ml DNase (Sigma) at 37°C. Cells were plated on poly-l-lysine-coated Petri dishes in F12 medium with 10% fetal bovine serum and examined 5 h after plating. For Ca2+ imaging experiments cells were incubated for 40 min at 20–22°C in physiological solution containing fluo-3 (AM ester cell-permeable compound; 1 μM; Invitrogen), followed by a 30-min washout period. Fluorescence in neurons was imaged using Cell-R imaging system (Olympus Europe, Hamburg, Germany). The imaging system consisted of the Olympus IX-70 inverted microscope equipped with a 175-W xenon burner light source. Fluorescence of fluo-3 was excited at 490 ± 5 nm and collected at 520 ± 15 nm. Images were collected in a time-lapse mode (2 Hz; 100 ms exposure time) with a CCD camera (Orca, Hamamatsu, Japan). To prevent photobleaching, illumination was used only during agonist application (with 10 min intervals). Fluorescent signals from single cells were quantified as the ΔF/F0, where F0 is the background subtracted baseline fluorescence and ΔF is the increment over the baseline). All drugs were applied via fast perfusion system (solution exchange time ~30 ms, RDS-200, Bio-Logic - Science Instruments, Grenoble, France). P2X3 receptors were activated with the selective agonist α,β-methylenATP (α,β-meATP, 10 μM) while TRPV1 receptors were activated with capsaicin (200 nM). Plots and figures were constructed using Origin software (OriginLab, version 8.0).
Single GABA channel recordings
Single GABA channel recordings were performed from the trigeminal sensory neurons prepared as described above. Cell-attached patch-clamp recordings were performed using EPC-9 amplifier (HEKA Elektronik Dr. Schulze GmbH, Lambrecht/Pfalz, Germany). Patch electrodes were made from borosilicate glass capillaries (GC150F-15, Clark Electromedical Instruments). For recordings of single GABA(A) channels, patch pipette solution contained (in mM): NaCl 120, TEA-Cl 20, KCl 5, 4-aminopyridine 5, CaCl2 0.1, MgCl2 10, glucose 10, HEPES–NaOH 10 buffered to pH 7.2–7.3 and GABA (1–5 μM) was added at the day of experiment from 1 mM frozen stock solution. Driving force for GABA(A) receptor mediated currents was determined from the current–voltage relationships of the currents through single GABA(A) channels single as described earlier (Tyzio et al., 2006) and corrected for an error of 2 mV (Tyzio et al., 2008).
Drugs
In the experiments in vivo, oxytocin (Sigma-Aldrich, St. Louis, MO, USA) 50 μM was injected at 0.1 ml/5g (diluted in saline) i.p., 30 min before testing. Bumetanide (Burinex, LEO pharmaceutical Products, Denmark) solution 25 μg/ml was injected i.p. at the dose of 5 μmol/kg, 30 min before testing. Atosiban (Sigma) (diluted in saline) was injected at 2 μg/kg, i.p., 30 min before testing. SSR126768A (gift from Sanofi-Synthelabo) diluted in saline injected at 1 μg/kg i.p, 30 min before testing. Sham injections in the control group were performed with equal volumes of saline.
Statistical analysis
Results are expressed as mean ± SEM. Data were analyzed by a two-way analysis of variance (ANOVA) followed, when the F value was significant, by a Fischer t-test, when the time-course of the effect was compared. Significance of changes in experiments with vocalization in vivo was tested by the Kruskal–Wallis test (H-test). In calcium imaging experiments significance was analyzed by non-parametric Mann–Whitney test. The level of statistical significance (*) was set at P < 0.05.
Results
In the present study, we used a combination of behavioral tests including thermal tail-flick and electrical stimulation evoked vocalizations, as well as electrophysiological and imaging approaches in isolated trigeminal neurons to study pain control by oxytocin in the newborn rats.
Analgesic Actions of Oxytocin with Thermal Tail-Flick Assay
We first tested pain sensitivity in the newborn rats using a thermal tail-flick response. In this test, pain sensitivity is reciprocal to the delay in tail withdrawal from the hot water. Previous developmental studies using this test indicated that nociceptive withdrawal thresholds are low in rat pups during the first postnatal week and only increase to adult values by the second or third postnatal week (Fitzgerald and Gibson, 1984; Falcon et al., 1996; Jiang and Gebhart, 1998; Teng and Abbott, 1998; Marsh et al., 1999). We studied thermal tail-flick responses in two age groups: (i) newborn animals within an hour after birth (P0) and (ii) 2-day-old rat pups (P2). Oxytocin levels are maximal during and immediately after birth, and wane during the first postnatal day, as deduced from the dynamic changes in GABA signaling in the cortical neurons (Tyzio et al., 2006). Under control conditions, P0 rats withdrew their tails within 4.7 ± 0.19 s (n = 15; Figure 1A). In P2 rats, delay in the tail withdrawal was of 2.4 ± 0.16 s (n = 15; Figure 1B), that is nearly two times shorter than P0 control rats (P < 0.0001). Thus, pain sensitivity in the newborn rats is significantly lower than in 2-day-old rats. To determine the contribution of endogenous oxytocin to this difference, we used selective blockers of oxytocin receptors atosiban (2 μg/kg, i.p.) and SSR126768A (1 μg/kg, i.p.). Both competitive antagonists caused a nearly three-fold acceleration of tail withdrawal in P0 pups; rendering them similar to that seen in P2 rats under control conditions (Figures 1A,B). In contrast, oxytocin receptor blockers did not significantly modify the tail-flick delays in P2 animals (Figure 1B). These findings suggest a strong analgesic effect of endogenous oxytocin in the newborn rats, and that this effect wanes with a postnatal reduction in oxytocin levels. Systemic administration of exogenous oxytocin (1 μg/kg) resulted in a dramatic analgesic effect both in newborn and P2 rats (Figures 1A,B). In the newborn rats, exogenous oxytocin could also partially reverse the effects of the competitive oxytocin receptor blockers, indicating that endogenous oxytocin levels are not saturated, and that therapeutic elevation of oxytocin levels could result in more powerful analgesia in the newborn.
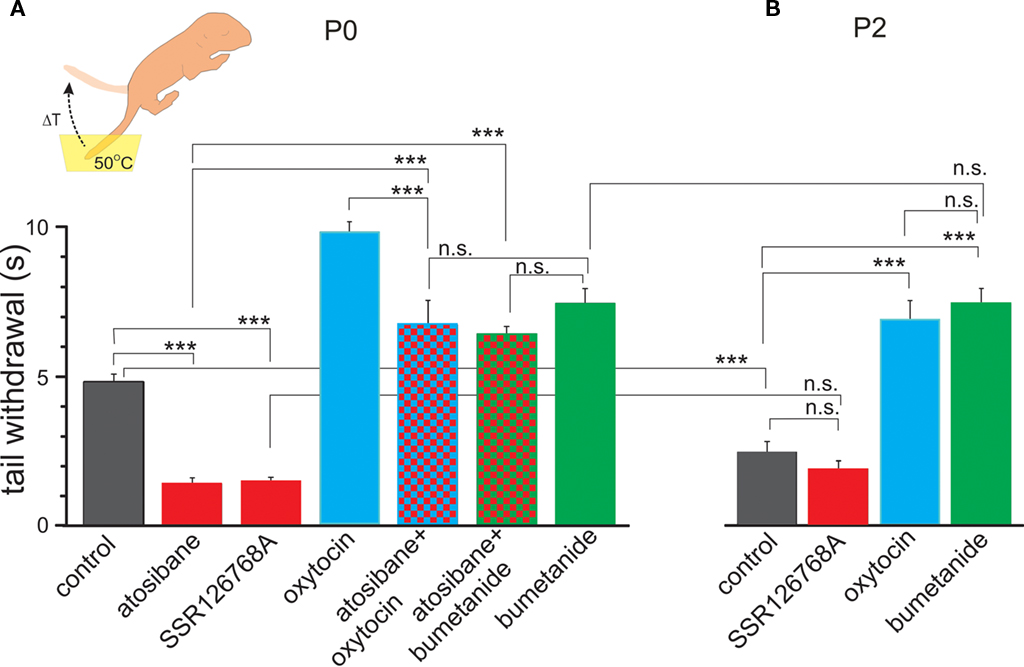
Figure 1. Oxytocin induces analgesia in newborn rat pups. Tail-flick pain response in the newborn (A) and 2-day-old (B) rat pups. Inset: schematic drawing of the tail-flick pain test, in which the pup’s tail is immersed in hot water (50°C) and the delay of tail withdrawal (ΔT) is used as a measure of pain. Note that in the newborn (P0) rats, oxytocin receptor blockers atosiban and SSR126768A strongly accelerate tail withdrawal, whereas oxytocin and the NKCC1 antagonist bumetanide alleviate pain. In 2-day-old rat pups, in which endogenous oxytocin levels waned, pain responses are much stronger than in the newborn rats and blockade of oxytocin receptors does not modify the response, however, exogenous oxytocin and bumetanide still exert powerful analgesic action (mean ± SEM; 5–15 animals for each column; ***P < 0.001; n.s., P > 0.05).
Analgesic Actions of Oxytocin with Pain-Induced Vocalization
As a second approach, we studied oxytocin-dependent modulation of trigeminal pain responses by measuring vocalization induced by electrical stimulation of the whisker pad. Animals were decerebrated at caudal midbrain levels (Figure 2A) to cut the descending oxytocin projections from the VPN and to prevent noxious input to the forebrain. Electrical stimulation of the whisker pad evoked vocalization in the neonatal rats despite this decerebration (Figure 2). Vocalizing responses were composed of several bursts with a dominant frequency in the range from 2.7 to 5 kHz (mean frequency 3.9 ± 0.1 kHz, n = 24, rat P0–2). To quantify vocalization response, we calculated a scalar sonogram integral. In agreement with the results of the thermal tail-flick assay, the oxytocin receptor blocker atosiban (2 μg/kg) increased vocalization responses in P0 rats (to 155 ± 28% of control values, n = 8, P = 0.0003; Figure 3A). In P1–2 rats, injections of saline did not change the vocalization response (105 ± 21% of control values; n = 6; P > 0.05). However, exogenous oxytocin strongly reduced vocal responses from 15.2 ± 6.4 to 8.2 ± 5.6 a.u., that is to 42 ± 12% (n = 6; P = 0.00004), and this effect was partially reversed by atosiban to 84 ± 38% from the control values (n = 6; P = 0.0017, Figures 2 and 3). Taken together, the results of both behavioral tests indicate that endogenous oxytocin reduces pain in newborn rats.
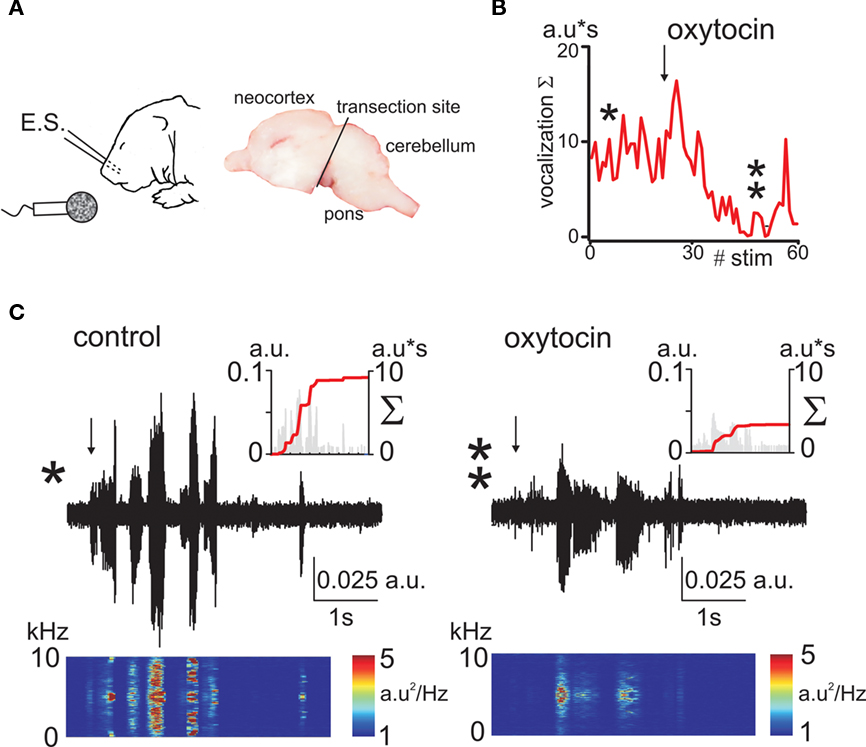
Figure 2. Oxytocin reduces electrical stimulation evoked vocalizations in decerebrated newborn rat pups. (A) Experimental setup. A train of electrical stimuli (E.S.) is delivered to the whisker pad, and the vocalizations are recorded by microphone. Right, photograph of the sagittal brain section showing the decerebration cut. (B) Time course of the effect of oxytocin on vocalizations (a.u = arbitrary unit). (C) Sonogram of the electrostimulation evoked vocalization in P1 pup in control conditions (left trace) and 20 min after administration of oxytocin (1 μmol/kg, i.p.; right trace). Spectrograms of the vocalization responses are shown under each trace. Insets show corresponding scalar vocalization response (gray bars, left ordinate) and scalar vocalization integral (red line, right ordinate).
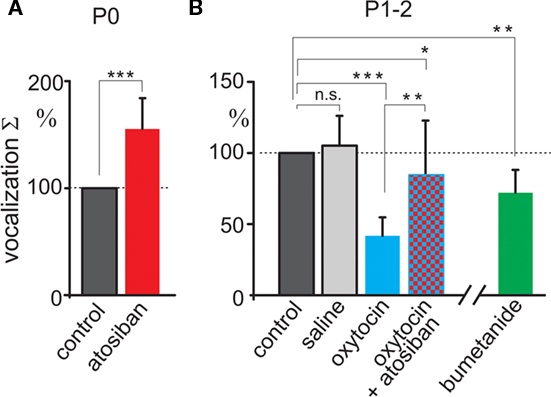
Figure 3. Summary plot of the oxytocin modulation of vocalization responses. Scalar vocalization integrals of the responses evoked by electrical whisker pad stimulation in decerebrated rat pups at P0 (A) and at P1–2 (B). Vocalization integrals are normalized to the control values. Note that vocalizations are enhanced by the oxytocin receptor antagonist atosiban in the newborn rats, and that they are reduced by exogenous oxytocin and NKCC1 blocker bumetanide in P1–2 rat pups. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., P > 0.05.
Analgesic Actions of Oxytocin are Mediated by GABA Signaling
Oxytocin induces a transient excitatory-to-inhibitory switch in the action of GABA on immature neurons at birth (Tyzio et al., 2006), and GABAergic mechanisms are implicated in the analgesic actions of oxytocin in adult animals (Condés-Lara et al., 2009). To examine the role of oxytocin-mediated regulation of GABA current polarity in neonatal analgesia, we used bumetanide – a selective blocker of NKCC1 co-transporter that, like oxytocin, lowers intracellular chloride and shifts the depolarizing/excitatory actions of GABA on immature neurons (Tyzio et al., 2006). Bumetanide (5 μmol/kg) strongly delayed the tail-flick responses in both age groups, and, importantly, prevented effects of oxytocin receptor blockers in newborn rats (Figure 1). Vocalizations induced in decerebrated pups were also reduced in P1–2 rats by bumetanide to 72 ± 16% of control (n = 6; P = 0.004, Figure 3). Taken together, these results suggest that endogenous oxytocin reduces pain in newborn rats by shifting the level of intracellular chloride that in turn changes the action of GABA in the pain circuits.
Oxytocin Modulates GABA Signaling in the Primary Nociceptive Neurons
As the actions of oxytocin in adult rats involve modulation of GABAergic control of the primary nociceptive afferents (Condés-Lara et al., 2009), we studied the effect of oxytocin on GABA responses in trigeminal sensory neurons, which detect noxious stimuli from the head and facial tissues and conduct them to the brainstem. Experiments were performed in primary cultures of trigeminal neurons dissociated from newborn rats and kept for 5 h in vitro. In keeping with previous observations (Reichling et al.,1994; Wang et al., 1994), activation of GABA receptors induced robust transient increases of intracellular calcium in trigeminal neurons, indicating a depolarizing action of GABA and calcium entry into the cells via voltage-gated calcium channels (Figure 4). Application of 1 μM oxytocin induced slow transient responses lasting ∼60–80 s (not shown) and significantly reduced GABA-evoked calcium transients. It is known that the depolarizing action of GABA in sensory neurons results from elevated intracellular chloride concentration, set by the highly expressed NKCC1 membrane chloride co-transporter (Delpire and Mount, 2002). In keeping with this observation, bumetanide suppressed GABA-evoked calcium transients like oxytocin confirming the importance of chloride and GABA in mediating these analgesic responses (Figures 4A,B).
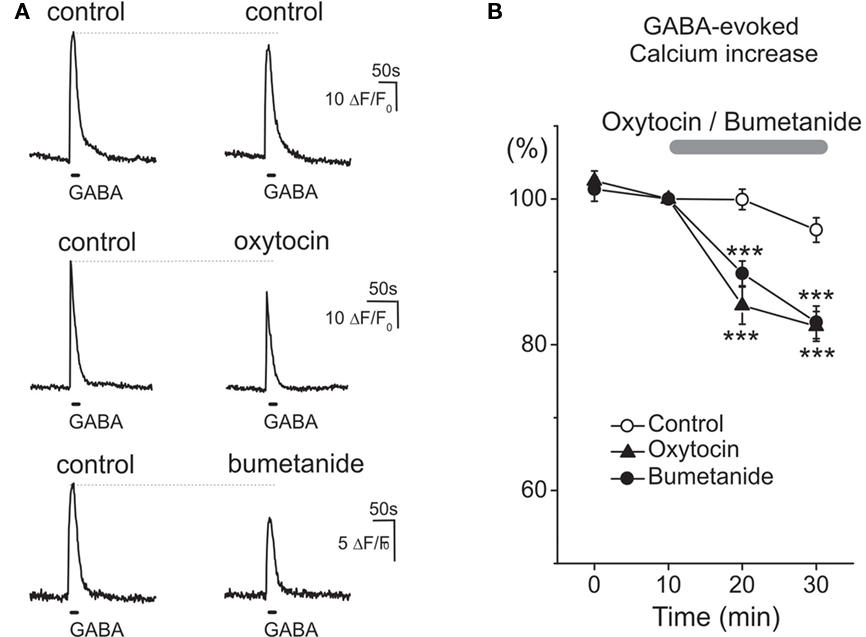
Figure 4. Oxytocin and bumetanide similarly reduce the depolarizing action of GABA on neonatal rat primary nociceptive neurons in vitro. (A) Examples of Ca2+ transients in isolated neonatal (P0) trigeminal sensory neurons induced by two sequential applications of GABA (100 μM at 30 min interval) in control conditions (top traces), and after 30 min exposure to 1 μM oxytocin (middle traces) and 10 μM bumetanide (bottom traces). (B) Summary plot shows the time course of changes in the normalized amplitude of GABA-induced Ca2+ transients in control conditions (n = 56 cells), and during application of oxytocin (n = 31 cells) and bumetanide (n = 15 cells).
To determine the specificity of oxytocin/GABA interactions in pain modulation, we tested the effects of the hormone on P2X3 receptors and TRPV1 receptors which represent two major types of pain transducers in trigeminal neurons (Simonetti et al., 2006). Figure 5 shows that repetitive (at 10 min intervals) 2 s long applications of the selective P2X3 receptor agonist α,β-meATP (n = 276 cells) and the TRPV1 agonist capsaicin (n = 223 cells) evoked transient, and essentially reproducible Ca2+ transients in neonatal trigeminal neurons. Application of 1 μM oxytocin for 20–30 min did not change the amplitude of these responses (n = 283 and 248 cells for α β-meATP and capsaicin, respectively). Thus, oxytocin does not modify P2X3 and TRPV1 receptor mediated responses in trigeminal neurons, further supporting our hypothesis that antinociceptive effects of oxytocin involve modulation of GABA signaling in the nociceptive neurons.
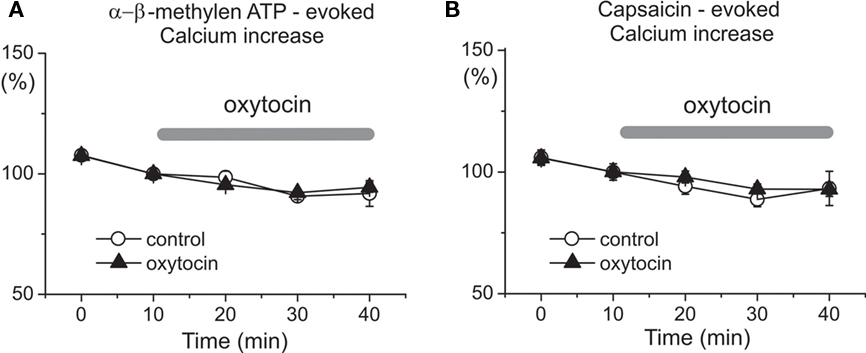
Figure 5. Oxytocin does not affect P2X and TRPV1 receptor mediated calcium transients in the neonatal rat primary nociceptive neurons in vitro. Dynamics of mean amplitudes of α,β-meATP (A) and capsaicin (B)-evoked calcium responses under control conditions (open circles; n = 276 and 223 cells for α,β-meATP and capsaicin, respectively) and during oxytocin application (triangles; n = 283 and 248 cells, respectively).
Because the results of calcium imaging suggest that oxytocin reduces the depolarizing action of GABA, we studied the effect of oxytocin on the GABA driving force (DFGABA) using cell-attached recordings of single GABA(A) channels (Figure 6). DFGABA was deduced from reversal potential of the currents via GABA(A) channels (Serafini et al., 1995; Tyzio et al., 2006, 2008). In control conditions, GABA exerted strongly depolarizing action on the immature trigeminal neurons with DFGABA of 38.7 ± 2.4 mV (n = 6). In the presence of oxytocin (1 μM), DFGABA was reduced to 17.7 ± 6.7 mV (n = 5; P < 0.05), indicating that oxytocin strongly diminishes the depolarizing action of GABA on neonatal trigeminal neurons via a reduction of intracellular chloride as described in neonatal cortical neurons (Tyzio et al., 2006).
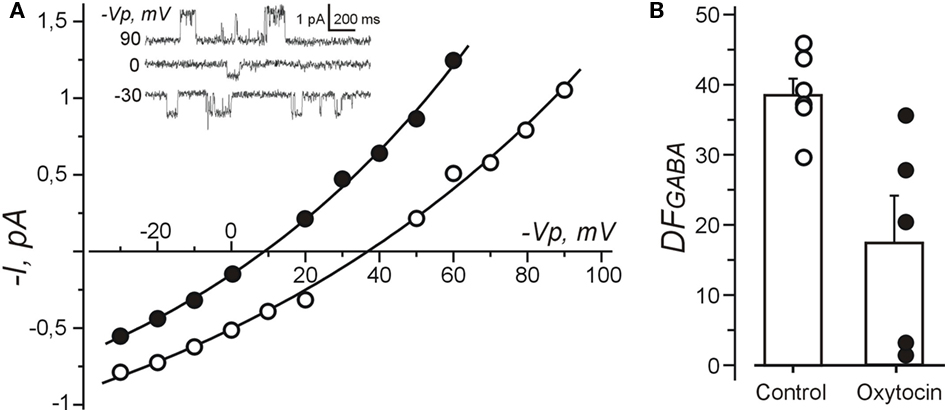
Figure 6. Oxytocin reduces depolarizing action of GABA measured using cell-attached recordings of single GABA channels from the neonatal rat primary nociceptive neurons in vitro. (A) Current–voltage relationships of the currents through single GABA channels recorded from isolated trigeminal neurons in vitro under control conditions and in the presence of oxytocin (1 μM). Examples of recordings at different pipette potentials (Vp) are shown on inset. (B) Summary plot shows the values of DFGABA in control conditions and in the presence of oxytocin. Each circle corresponds to individual neuron. Note that oxytocin reduces DFGABA in trigeminal neurons.
Discussion
Our results indicate that like human neonates (Bergqvist et al., 2008), newborn rats are born with a natural analgesia. Firstly, pain sensitivity in the thermal tail-flick assay was significantly lower in the newborn rats compared to P2 rats. This difference is unlikely due to a rapid developmental increase in the pain sensitivity during this short period because developmental studies indicate that pain sensitivity only reduces with age (Fitzgerald and Gibson, 1984; Falcon et al., 1996; Jiang and Gebhart, 1998; Teng and Abbott, 1998; Marsh et al., 1999), but rather reflects a birth-related phenomenon. Secondly, blockade of oxytocin receptors that did not significantly modify the response in P2 rats, when birth-derived oxytocin levels have waned, but did strongly increase pain sensitivity in the rats immediately after birth. The present results are in agreement with clinical findings of newborn analgesia in vaginal delivery when compared to elective cesarean sections without labor (Bergqvist et al., 2008). Relying on this observation, it has been proposed that the fetus exhibits a high pain threshold during delivery, and that this threshold decreases significantly within hours after spontaneous vaginal birth. Thus, newborn analgesia exists both in human and rodent suggesting that it is a fundamental mechanism developed and conserved through the mammalian evolution. The physiological role for the newborn analgesia is likely to prevent newborns from experiencing the pain inherent in the mechanical compression in the delivery channel. Indeed, during delivery the human newborn’s head is squeezed with an intermittent pressure as high as 2 kg/cm−2 for several hours (Derek, 1999). However, it should equally alleviate pain resulting from iatrogenic factors, including forceps and vacuum extraction, placement of electrodes, blood samples during labor, and injections of vitamins and immunization. In keeping with the time course of newborn analgesia, which wanes within few hours, it has been recommended that painful procedures such as vitamin K injection or blood sampling to be performed within 90 min after birth in order to minimize neonatal pain and possible long-lasting changes in pain behavior (Bergqvist et al., 2008).
What are the mechanisms of newborn analgesia? Because delivery is associated with stress, and because stress induces analgesia, it likely stress-induced noradrenalin and opioids releases mediate newborn analgesia (Bistoletti et al., 1983; Hagnevik et al., 1984; Lagercrantz and Slotkin, 1986; Lagercrantz, 1996; Mogil et al., 1996). The results of the present study also indicate an involvement of oxytocin, who’s analgesic actions have been well documented in adult animals (Arletti et al., 1993; Lundeberg et al., 1993, 1994; Agren et al., 1995; Petersson et al., 2001; Yu et al., 2003; Condés-Lara et al., 2006; Miranda-Cardenas et al., 2006). Indeed, blockade of oxytocin receptors significantly increased pain in both assays in the newborn animals, suggesting an involvement of endogenous oxytocin. Conversely, exogenous oxytocin exerted analgesic actions in the animals newborn and in 1- to 2-day-old rat pups, when endogenous oxytocin has waned. Thus, newborn analgesia is likely achieved by activation of several complementary antinociceptive stress-induced (catecholamines and opioids) and birth-related (oxytocin) mechanisms to optimally protect newborn from the pain. Although the relative contributions of these various mechanisms to newborn analgesia remain to be determined, the powerful proalgesic effects of the oxytocin receptor antagonists in the newborn rats indicate that endogenous oxytocin provides a significant contribution to newborn analgesia.
What is the source for oxytocin in the newborn? In the fetal brain, oxytocin may be provided both by the mother and the fetus, and the maternal and fetal contributions may differ in rodents and humans. In the newborn rat the principal source of oxytocin is maternal, with only a limited contribution of fetus. This is because the hypothalamo-neurohypophysial system develops mainly post partum (Choy and Watkins, 1979; Lipari et al., 2001) and the production of fully functional amidated oxytocin begins only after birth and increases rapidly during postnatal development (Alstein et al., 1988; Lipari et al., 2001). Thus, the onset of intrinsic oxytocin production does not match with the observed perinatal oxytocin-mediated changes in GABA signaling, which is already half-maximal by E20 (Tyzio et al., 2006), nor with the analgesic actions of endogenous oxytocin at birth. In addition, we found that blockade of oxytocin receptors exerted pro-nociceptive action in the decerebrated newborn rats in which the descending oxytocin fibers from PVN and the upstream pain pathways are cut. Taken together, these results indicate that oxytocin in the newborn rat brain is mainly provided by the mother.
Whether our findings on the perinatal analgesic action of oxytocin can be translated to human newborn remains unknown. However such a hypothesis is supported by the findings of higher pain threshold in the vaginally born compared to cesarean-born newborns (Bergqvist et al., 2008), elevated oxytocin levels under normal delivery but not during C-sections (Russell et al., 2003; Wei et al., 2009), and by the analgesic effects of oxytocin in humans (Yang, 1976). On the other hand, the level of maturity of human brain at term is more advanced compared with altricial rats (Clancy et al., 2001) and nociceptive systems undergo significant changes during development (Fitzgerald and Gibson, 1984; Falcon et al., 1996; Jiang and Gebhart, 1998; Teng and Abbott, 1998; Marsh et al., 1999). In regards to the source of oxytocin, the contribution of fetal oxytocin to perinatal analgesia may be more significant in humans as their hypothalamo-neurohypophyseal system is more advanced at term and is capable of producing proper oxytocin. Thus, in humans, oxytocin levels at birth are higher in the umbilical artery than in the umbilical vein (Chard et al., 1971; Dawood et al., 1978; Otsuki et al., 1983; Patient et al., 1999). In addition, no oxytocin was detected in either arterial or venous cord blood of anencephalic newborns (Chard et al., 1971; Otsuki et al., 1983). This may suggest that oxytocin-mediated newborn analgesia in the rat is mainly provided by mother oxytocin whereas in human, it is mainly provided by fetal oxytocin.
How does oxytocin exert an analgesic action? In adult rats, electrical stimulation of the PVN, or topical application of oxytocin, selectively inhibits A-delta and C-fiber responses in superficial dorsal horn neurons, and this inhibition is reversed by a selective oxytocin antagonists (Robinson et al., 2002; Condés-Lara et al., 2006; Rojas-Piloni et al., 2007, 2008). These effects are blocked by the GABA(A) receptor antagonist bicuculline, suggesting that the analgesic actions of oxytocin involve enhancement of presynaptic GABAergic inhibition of the primary nociceptive inputs conveyed by A-delta and C-fibers to the spinal cord (Condés-Lara et al., 2009). This is in keeping with a preferential expression of oxytocin receptors in the superficial layers of the dorsal spinal cord, which is already high at birth (Uhl-Bronner et al., 2005).
An increase in GABAergic inhibition of the primary nociceptive afferents likely contributes to the analgesic actions of oxytocin observed in the present study in the newborn rats. However, in addition to the increase in the amount of GABA release caused by increased interneuron firing, a change in EGABA seems to contribute to the oxytocin-mediated increase in GABAergic inhibition. Indeed, we found that oxytocin reduces DFGABA and reduces GABA-evoked calcium transients in the trigeminal neurons. EGABA shifts toward more depolarizing values in different models of chronic pain in adult animals (for reviews De Koninck, 2007; Price et al., 2009). Blockade of NKCC1 co-transporter, which is the primary mediator of chloride accumulation in primary nociceptive neurons with bumetanide, causes a negative shift in EGABA and reduces pain (Alvarez-Leefmans et al., 1988; Sung et al., 2000; Laird et al., 2004; Granados-Soto et al., 2005; Valencia-de Ita et al., 2006). Our findings that bumetanide exerts an analgesic action in rat pups, and most notably, that its analgesic action persists after blockade of oxytocin receptors, provides additional support to our hypothesis that changes in EGABA in the nociceptive neurons are involved in the newborn analgesia and analgesic actions of oxytocin. Because depolarizing action of GABA on primary nociceptive neurons persists through the development (Alvarez-Leefmans et al., 1988; Sung et al., 2000; Gilbert et al., 2007; Rocha-González et al., 2008), it would be of interest to determine whether similar changes in EGABA contribute to oxytocin-mediated analgesia in adults, and notably in parturient women.
In conclusion, our results indicate that (i) like human neonates (Bergqvist et al., 2008), newborn rats are born with a natural analgesia, that (ii) this newborn analgesia involves the antinociceptive effects of endogenous oxytocin, and that (iii) the antinociceptive action of oxytocin involves modulation of GABA signaling in the nociceptive neurons. This reveals a novel facet of the protective actions of oxytocin for the newborn, which include previously described role in the transient switch in cortical actions of GABA from excitatory-to-inhibitory and an increase in brain resistance to hypoxia (Tyzio et al., 2006; Khazipov et al., 2008). In future clinical studies it would be of interest to verify the role for oxytocin in perinatal analgesia in the human newborn.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Financial support from INSERM, ANR, FRM, LFCE, Rothschild/INSERM “interface” programs to Rustem Khazipov and Yehezkel Ben-Ari. Anastasia Shakirzyanova and Rashid Giniatullin were supported by Finnish Academy.
References
Agren, G., Lundeberg, T., Uvnäs-Moberg, K., and Sato, A. (1995). The oxytocin antagonist 1-deamino-2-D-Tyr-(Oet)-4-Thr-8-Orn-oxytocin reverses the increase in the withdrawal response latency to thermal, but not mechanical nociceptive stimuli following oxytocin administration or massage-like stroking in rats. Neurosci. Lett. 187, 49–52.
Alstein, M., Whitnall, M. H., House, S., Key, S., and Gainer, H. (1988). An immunochemical analysis of oxytocin and vasopressin prohormone processing in vivo. Peptides 9, 87–105.
Alvarez-Leefmans, F. J., Gamino, S. M., Giraldez, F., and Nogueron, I. (1988). Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J. Physiol. (Lond.) 406, 225–246.
Anand, K. J. (2001). Consensus statement for the prevention and management of pain in the newborn. Arch. Pediatr. Adolesc. Med. 155, 173–180.
Anand, K. J., and Hickey, P. R. (1987). Pain and its effects in the human neonate and fetus. N. Engl. J. Med. 317, 1321–1329.
Argiolas, A., and Gessa, G. L. (1991). Central functions of oxytocin. Neurosci. Biobehav. Rev. 15, 217–231.
Arletti, R., Benelli, A., and Bertolini, A. (1993). Influence of oxytocin on nociception and morphine antinociception. Neuropeptides 24, 125–129.
Bergqvist, L. L., Katz-Salamon, M., Hertegard, S., Anand, K. J. S., and Lagercrantz, H. (2008). Mode of delivery modulates physiological and behavioral responses to neonatal pain. J. Perinatol. 29, 44–50.
Bistoletti, P., Nylund, L., Lagercrantz, H., Hjemdahl, P., and Strom, H. (1983). Fetal scalp catecholamines during labor. Am. J. Obstet. Gynecol. 147, 785–788.
Chard, T., Hudson, C. N., Edwards, C. R., and Boyd, N. R. (1971). Release of oxytocin and vasopressin by the human foetus during labour. Nature 234, 352–354.
Choy, V. J., and Watkins, W. B. (1979). Maturation of the hypothalamo-neurohypophysial system. I. Localization of neurophysin, oxytocin and vasopressin in the hypothalamus and neural lobe of the developing rat brain. Cell Tissue Res. 197, 325–336.
Clancy, B., Darlington, R. B., and Finlay, B. L. (2001). Translating developmental time across mammalian species. Neuroscience 105, 7–17.
Condés-Lara, M., Rojas-Piloni, G., Martinez-Lorenzana, G., Lopez-Hidalgo, M., and Rodriguez-Jimenez, J. (2009). Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 1247, 38–49.
Condés-Lara, M., Rojas-Piloni, G., Martínez-Lorenzana, G., Rodríguez-Jiménez, J., López Hidalgo, M., and Freund-Mercier, M. J. (2006). Paraventricular hypothalamic influences on spinal nociceptive processing. Brain Res. 1081, 126–137.
Dawood, M. Y., Wang, C. F., Gupta, R., and Fuchs, F. (1978). Fetal contribution to oxytocin in human labor. Obstet. Gynecol. 52, 205–209.
De Koninck, Y. (2007). Altered chloride homeostasis in neurological disorders: a new target. Curr. Opin. Pharmacol. 7, 93–99.
Delpire, E., and Mount, D. B. (2002). Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu. Rev. Physiol. 64, 803–843.
Falcon, M., Guendellman, D., Stolberg, A., Frenk, H., and Urca, G. (1996). Development of thermal nociception in rats. Pain 67, 203–208.
Fitzgerald, M., and Gibson, S. (1984). The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience 13, 933–944.
Gilbert, D., Franjic-Würtz, C., Funk, K., Gensch, T., Frings, S., and Möhrlen, F. (2007). Differential maturation of chloride homeostasis in primary afferent neurons of the somatosensory system. Int. J. Dev. Neurosci. 25, 479–489.
Gimpl, G., and Fahrenholz, F. (2001). The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 81, 629–683.
Granados-Soto, V., Arguelles, C. F., and Alvarez-Leefmans, F. J. (2005). Peripheral and central antinociceptive action of Na+-K+-2Cl− cotransporter blockers on formalin-induced nociception in rats. Pain 114, 231–238.
Hagnevik, K., Faxelius, G., Irestedt, L., Lagercrantz, H., Lundell, B., and Persson, B. (1984). Catecholamine surge and metabolic adaptation in the newborn after vaginal delivery and caesarean section. Acta Paediatr. Scand. 73, 602–609.
Huber, D., Veinante, P., and Stoop, R. (2005). Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248.
Jiang, M. C., and Gebhart, G. F. (1998). Development of mustard oil-induced hyperalgesia in rats. Pain 77, 305–313.
Jourdan, D., Ardid, D., Chapuy, E., Eschalier, A., and Le, B. D. (1995). Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to the tail in the rat. Pain 63, 237–249.
Jourdan, D., Ardid, D., Chapuy, E., Le, B. D., and Eschalier, A. (1997). Audible and ultrasonic vocalization elicited by a nociceptive stimulus in rat: relationship with respiration. J. Pharmacol. Toxicol. Methods 38, 109–116.
Khazipov, R., Tyzio, R., and Ben Ari, Y. (2008). Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog. Brain Res. 170, 243–257.
Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U., and Fehr, E. (2005). Oxytocin increases trust in humans. Nature 435, 673–676.
Lagercrantz, H. (1996). Stress, arousal, and gene activation at birth. News Physiol. Sci. 11, 214–218.
Lagercrantz, H., and Bistoletti, P. (1977). Catecholamine release in the newborn infant at birth. Pediatr. Res. 11, 889–893.
Laird, J. M., García-Nicas, E., Delpire, E. J., and Cervero, F. (2004). Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci. Lett. 361, 200–203.
Laprairie, J. L., and Murphy, A. Z. (2009). Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front. Behav. Neurosci. 3:31. doi: 10.3389/neuro.08.031.2009
Lipari, E. F., Lipari, D., Gerbino, A., Di Liberto, D., Bellafiore, M., Catalano, M., and Valentino, B. (2001). The hypothalamic magnocellular neurosecretory system in developing rats. Eur. J. Histochem. 45, 163–168.
Lundeberg, T., Meister, B., Björkstrand, E., and Uvnäs-Moberg, K. (1993). Oxytocin modulates the effects of galanin in carrageenan-induced hyperalgesia in rats. Brain Res. 608, 181–185.
Lundeberg, T., Uvnäs-Moberg, K., Agren, G., and Bruzelius, G. (1994). Anti-nociceptive effects of oxytocin in rats and mice. Neurosci. Lett. 170, 153–157.
Marsh, D., Dickenson, A., Hatch, D., and Fitzgerald, M. (1999). Epidural opioid analgesia in infant rats I: mechanical and heat responses. Pain 82, 23–32.
Miranda-Cardenas, Y., Rojas-Piloni, G., Martínez-Lorenzana, G., Rodríguez-Jiménez, J., López-Hidalgo, M., Freund-Mercier, M. J., and Condés-Lara, M. (2006). Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain 122, 182–189.
Mogil, J. S., Sternberg, W. F., Balian, H., Liebeskind, J. C., and Sadowski, B. (1996). Opioid and nonopioid swim stress-induced analgesia: a parametric analysis in mice. Physiol. Behav. 59, 123–132.
Otsuki, Y., Tanizawa, O., Yamaji, K., Fujita, M., and Kurachi, K. (1983). Feto-maternal plasma oxytocin levels in normal and anencephalic pregnancies. Acta Obstet. Gynecol. Scand. 62, 235–237.
Patient, C., Davison, J. M., Charlton, L., Baylis, P. H., and Thornton, S. (1999). The effect of labour and maternal oxytocin infusion on fetal plasma oxytocin concentration. Br. J. Obstet. Gynaecol. 106, 1311–1313.
Petersson, M., Wiberg, U., Lundeberg, T., and Uvnäs-Moberg, K. (2001). Oxytocin decreases carrageenan induced inflammation in rats. Peptides 22, 1479–1484.
Pieraut, S., Laurent-Matha, V., Sar, C., Hubert, T., Mechaly, I., Hilaire, C., Mersel, M., Delpire, E., Valmier, J., and Scamps, F. (2007). NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons. J. Neurosci. 27, 6751–6759.
Price, T. J., Cervero, F., Gold, M. S., Hammond, D. L., and Prescott, S. A. (2009). Chloride regulation in the pain pathway. Brain Res. Rev. 60, 149–170.
Raggenbass, M. (2001). Vasopressin- and oxytocin-induced activity in the central nervous system: electrophysiological studies using in-vitro systems. Prog. Neurobiol. 64, 307–326.
Reichling, D. B., Kyrozis, A., Wang, J., and MacDermott, A. B. (1994). Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J. Physiol. (Lond.) 476, 411–421.
Robinson, D. A., Wei, F., Wang, G. D., Li, P., Kim, S. J., Vogt, S. K., Muglia, L. J., and Zhuo, M. (2002). Oxytocin mediates stress-induced analgesia in adult mice. J. Physiol. (Lond.) 540, 593–606.
Rocha-González, H. I., Mao, S., and Alvarez-Leefmans, F. J. (2008). Na+,K+,2Cl− cotransport and intracellular chloride regulation in rat primary sensory neurons: thermodynamic and kinetic aspects. J. Neurophysiol. 100, 169–184.
Rojas-Piloni, G., López-Hidalgo, M., Martínez-Lorenzana, G., Rodríguez-Jiménez, J., and Condés-Lara, M. (2007). GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 1137, 69–77.
Rojas-Piloni, G., Martinez-Lorenzana, G., DelaTorre, S., and Condés-Lara, M. (2008). Nociceptive spinothalamic tract and postsynaptic dorsal column neurons are modulated by paraventricular hypothalamic activation. Eur. J. Neurosci. 28, 546–558.
Russell, J. A., Leng, G., and Douglas, A. J. (2003). The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front. Neuroendocrinol. 24, 27–61.
Serafini, R., Valeyev, A. Y., Barker, J. L., and Poulter, M. O. (1995). Depolarizing GABA-activated Cl− channels in embryonic rat spinal and olfactory bulb cells. J. Physiol. (Lond.) 488, 371–386.
Simonetti, M., Fabbro, A., D’Arco, M., Zweyer, M., Nistri, A., Giniatullin, R., and Fabbretti, E. (2006). Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Mol. Pain 2, 11.
Slater, R., Cornelissen, L., Fabrizi, L., Patten, D., Yoxen, J., Worley, A., Boyd, S., Meek, J., and Fitzgerald, M. (2010). Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet 376, 1225–1232.
Sternberg, W. F., Scorr, L., Smith, L. D., Ridgway, C. G., and Stout, M. (2005). Long-term effects of neonatal surgery on adulthood pain behavior. Pain 113, 347–353.
Sung, K.-W., Kirby, M., McDonald, M. P., Lovinger, D. M., and Delpire, E. (2000). Abnormal GABAA receptor mediated currents in dorsal root ganglion neurons isolated from Na-K-2l cotransport null mice. J. Neurosci. 20, 7531–7508.
Teng, C. J., and Abbott, F. V. (1998). The formalin test: a dose–response analysis at three developmental stages. Pain 76, 337–347.
Theodosis, D. T., Koksma, J. J., Trailin, A., Langle, S. L., Piet, R., Lodder, J. C., Timmerman, J., Mansvelder, H., Poulain, D. A., Oliet, S. H., and Brussaard, A. B. (2006). Oxytocin and estrogen promote rapid formation of functional GABA synapses in the adult supraoptic nucleus. Mol. Cell. Neurosci. 31, 785–794.
Tomizawa, K., Iga, N., Lu, Y. F., Moriwaki, A., Matsushita, M., Li, S. T., Miyamoto, O., Itano, T., and Matsui, H. (2003). Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat. Neurosci. 6, 384–390.
Tyzio, R., Cossart, R., Khalilov, I., Minlebaev, M., Hubner, C. A., Represa, A., Ben Ari, Y., and Khazipov, R. (2006). Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 314, 1788–1792.
Tyzio, R., Minlebaev, M., Rheims, S., Ivanov, A., Jorquera, I., Holmes, G. L., Zilberter, Y., Ben Ari, Y., and Khazipov, R. (2008). Postnatal changes in somatic gamma-aminobutyric acid signalling in the rat hippocampus. Eur. J. Neurosci. 27, 2515–2528.
Uhl-Bronner, S., Waltisperger, E., Martínez-Lorenzana, G., Condes Lara, M., and Freund-Mercier, M. J. (2005). Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience 135, 147–154.
Valencia-de Ita, S., Lawand, N. B., Lin, Q., Castaneda-Hernandez, G., and Willis, W. D. (2006). Role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. J. Neurophysiol. 95, 3553–3561.
Wang, J., Reichling, D. B., Kyrozis, A., and MacDermott, A. B. (1994). Developmental loss of GABA- and glycine-induced depolarization and Ca2+ transients in embryonic rat dorsal horn neurons in culture. Eur. J. Neurosci. 6, 1275–1280.
Wei, S. Q., Luo, Z. C., Xu, H., and Fraser, W. D. (2009). The effect of early oxytocin augmentation in labor: a meta-analysis. Obstet. Gynecol. 114, 641–649.
Yang, J. (1976). Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine (Phila Pa 1976) 19, 867–871.
Keywords: GABA, neonate, oxytocin, pain
Citation: Mazzuca M, Minlebaev M, Shakirzyanova A, Tyzio R, Taccola G, Janackova S, Gataullina S, Ben-Ari Y, Giniatullin R and Khazipov R (2011) Newborn analgesia mediated by oxytocin during delivery. Front. Cell. Neurosci. 5:3. doi:10.3389/fncel.2011.00003
Received: 31 January 2011;
Accepted: 31 March 2011;
Published online: 12 April 2011.
Edited by:
Enrico Cherubini, International School for Advanced Studies, ItalyReviewed by:
Heiko J. Luhmann, Institut für Physiologie und Pathophysiologie, GermanyHugo Lagercrantz, Karolinska Institutet, Sweden
Copyright: © 2011 Mazzuca, Minlebaev, Shakirzyanova, Tyzio, Taccola, Janackova, Gataullina, Ben-Ari, Giniatullin and Khazipov. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Rustem Khazipov, INMED/INSERM U901, 163 Route de Luminy, 13273 Marseille, France. e-mail: khazipov@inmed.univ-mrs.fr