Background: Vascular stents present the most common means for treating the severe cases of the coronary artery disease. However, restenosis and thrombosis are the major complications that are the major limitations of this technique. The continuous release of nitric oxide (NO) by native endothelium of blood vessel plays a substantial role in the cardiovascular physiology, suppression of vascular smooth muscle cell (VSMC) proliferation, and inhibition of platelet activation and aggregation.
Therefore, these unique advantages of NO make NO-releasing or -generating stent coatings very promising to perfectly meet the local requirements of an ideal stent concerning the blood vessel wall and the blood phase. To reduce the possibility of restenosis and thrombosis, this work focus on developing a durable NO-generating coating that mimics the endothelium functionality to improve the biocompatibility of vascular stents.
Materials and Methods: 316L stainless steel (SS) stent was firstly modified by a plasma polymerized allylamine (PPAam) coating rich in primary amine groups.[1] Then, the SeDPA was immobilized on PPAam-coated 316L SS stent in water-soluble carbodiimide (WSC) solution (pH 5.4) containing 0.5 mg/mL of SeDPA.[1]
Results and Discussion: The generated NO by SeDPA-PPAam surface remarkably increased the cGMP synthesis both in platelets and human umbilical artery smooth muscle cells (HUASMCs). The surface exhibited a remarkable suppression of collagen-induced platelet activation and aggregation, as well as HUASMC adhesion, proliferation and migration. Additionally, it was found that the SeDPA-PPAam surface significantly enhanced human umbilical vein endothelial cell (HUVEC) adhesion, proliferation and migration. It was highlighted that the NO catalytic surface created a favorable microenvironment of competitive growth of HUVECs over HUASMCs for promoting re-endothelialization and resulting in the reduction in the restenosis and thrombosis of stent (Fig. 1).
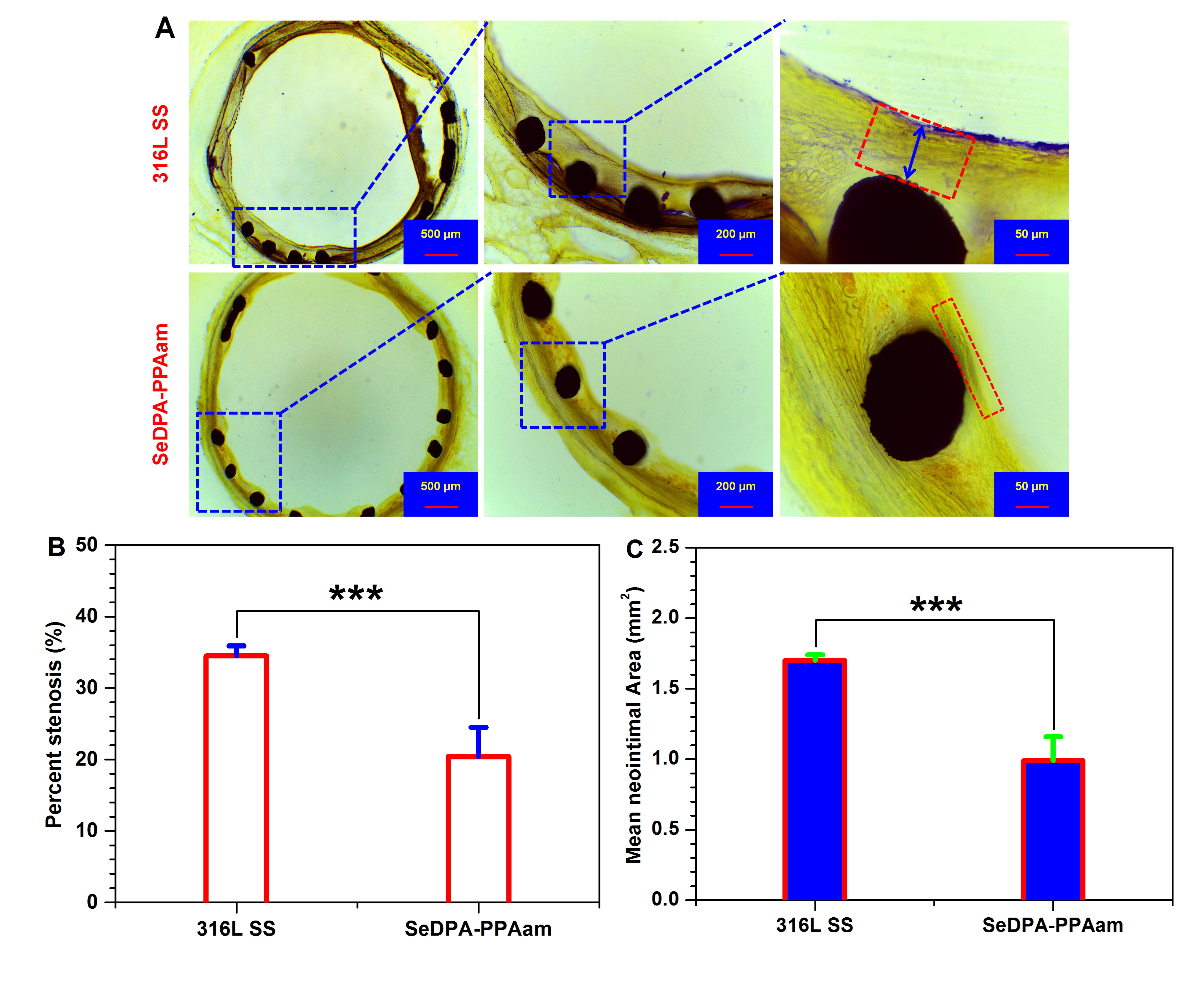
Fig. 1. (A) Representative cross-sectional images of htoxylin eosin stained iliac arteries with uncoated bare metal stent and stent coated with SeDPA-PPAam at week 4 after stent deployment. (B) Neointimal stenosis and (C) mean neointimal area analysis at the stented regions. Data presented as mean ± SD (n=3) and analyzed using a one–way ANOVA, ***p < 0.001.
Conclusion: Functionalization of 316L SS stent with NO-producing coating provided a native endothelium-mimetic microenvironment by long-term and continuous generation of NO, which is prone to promote the recovery of endothelium on the luminal surface of the stent and address the long term complications restenosis and thrombosis.
National Natural Science Foundation of China (Project 81271701); National Natural Science Foundation of China (Project 51173149); National Natural Science Foundation of China (Project 31270020); National Natural Science Foundation of China (Project 31300787); The Ministry of Science and Technology of China (Key Basic Research Project No. 2011CB606204); National Natural Science Foundation of China Key Program 81330031
References:
[1] Yang ZL, Tu QF, Maitz MF, Zhou S, Wang J, Huang N. Biomaterials 2012;33:7959-71.