Introduction: Compared with traditional medical stainless steel, high-nitrogen nickel-free stainless steel (HNS) without addition of hazardous Ni element shows excellent biocompatibility, mechanical properties and resistance to localized corrosion[1],[2]. Especially, its better blood compatibility is expected to improve the clinical performance for manufacturing coronary stents[3]. This study was focused on the preclinical in vivo evaluation of a novel kind of coronary stents made of HNS, Fe-17Cr-15Mn-2Mo-N. Both bio-safety and effectiveness of high-nitrogen nickel-free stainless steel stents with and without drug-eluting coating in the porcine coronary arteries were investigated, and other two kinds of stents that are available on applications, including naked 316L stainless steel stent and drug-eluting L605 (CoCrMo) alloy stent, were chosen as the control groups. In the study, quantitative histomorphometry, semi-quantitative histopathology, stent thrombosis, arterial injury, inflammation and statistical analysis were conducted at each chosen time point.
Materials and Methods: All the stents used in study were balloon-expandable coronary stents with size of 3.0 x15mm implanted into 48 pigs. Experimental group-1 was a bare metal stent (BMS) made of HNS, control group-1 was a commercial available BMS made of 316L stainless steel, experimental group-2 was a sirolimus drug eluting stent (DES) made of HNS, and control group-2 was a commercial DES made of L605 alloy. One experimental and its control group stents were implanted into two arteries of one pig.
After 14, 28 and 90 days implantation, the bio-safety evaluated by observation of restenosis and thrombosis on stent segments and in-stent restenosis, endothelialization of vessel and long-term safety were examined. The value of P<0.05 was considered statistically significant.
Results and Discussion: At 14 days implantation, there was a case of thrombosis happening in one pig with a control DES, at the meanwhile, the stent struts were not endothelial covered completely and there was some amount of inflammatory cells infiltration. Furthermore other stent samples, especially those high-nitrogen nickel-free stainless steel stents displayed well endothelialization, mild inflammatory and without mural thrombus, .
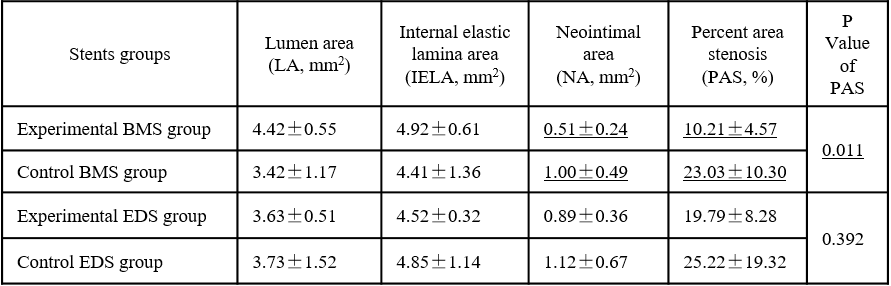
At each endpoints, experimental groups including BMS and DES were superior to their each control groups on the degree of intimal hyperplasia, and the experimental BMS had an significant advantage over the control BMS after 90 days implantation, as shown in Fig. 1 and Table 1 (P<0.05). How experimental stents exhibited such advantage over control groups should be that there was almost no Ni2+ released from HNS. Ni2+ could increase the levels of hypoxia inducible factor protein-1α (HIF-1α) and its target genes in the cultured smooth muscle cells[4], leading to more inflammatory and percentage area stenosis.
Conclusion: Experimental drug eluting stent (DES) system is a new type of coronary stents made of HNS. Compared with the commercial stents, HNS stent showed better biocompatibility with no thrombosis happening and lower mean value of diameter stenosis at early-term, displaying significant advantage on the degree of intimal hyperplasia at long-term implantation endpoint.
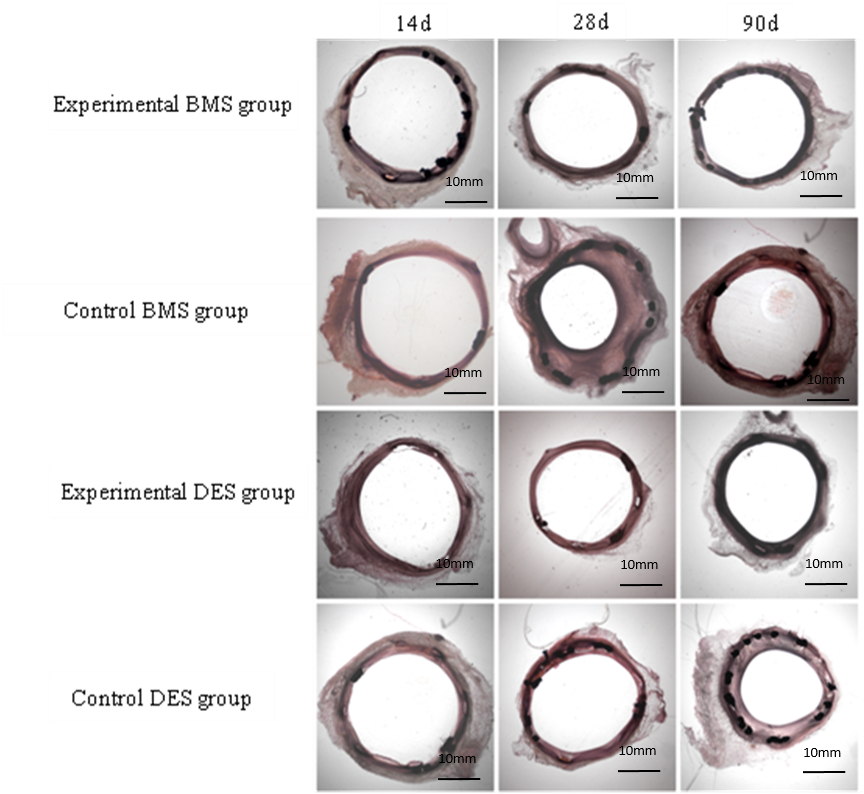
Fig.1 Histopathological photomicrographs of stented coronary artery of different groups
Table1 Quantitative coronary histomorphometry of each stent group after 90 days implantation
References:
[1] G. Stein, J. Menzel, Int. J. Mater. Prod. Tech., 10 (3-6) (1995), 290, Switzerland.
[2] Y.B. Ren, K. Yang, B.C. Zhang, Material Letters, 59 (14-15) (2005), 1785.
[3] P. Wan et al. / Materials Science and Engineering C 30 (2010) 1183–1189.
[4] Katsuhito Fujiu et al. Sci. Technol. Adv. Mater. 13 (2012) 064218 (10pp).