Introduction: We present a microengineered 3D hydrogel platform for culture of myelinated embryonic peripheral neural tissue as shown in Figure 1. This novel in-vitro platform enables morphological and physiological metrics analogous to clinical nerve fiber density and nerve conduction tests. We demonstrate the feasibility of this model system for studying peripheral neuropathy caused by glucose-induced neurotoxicity, the most common form of neuropathy.
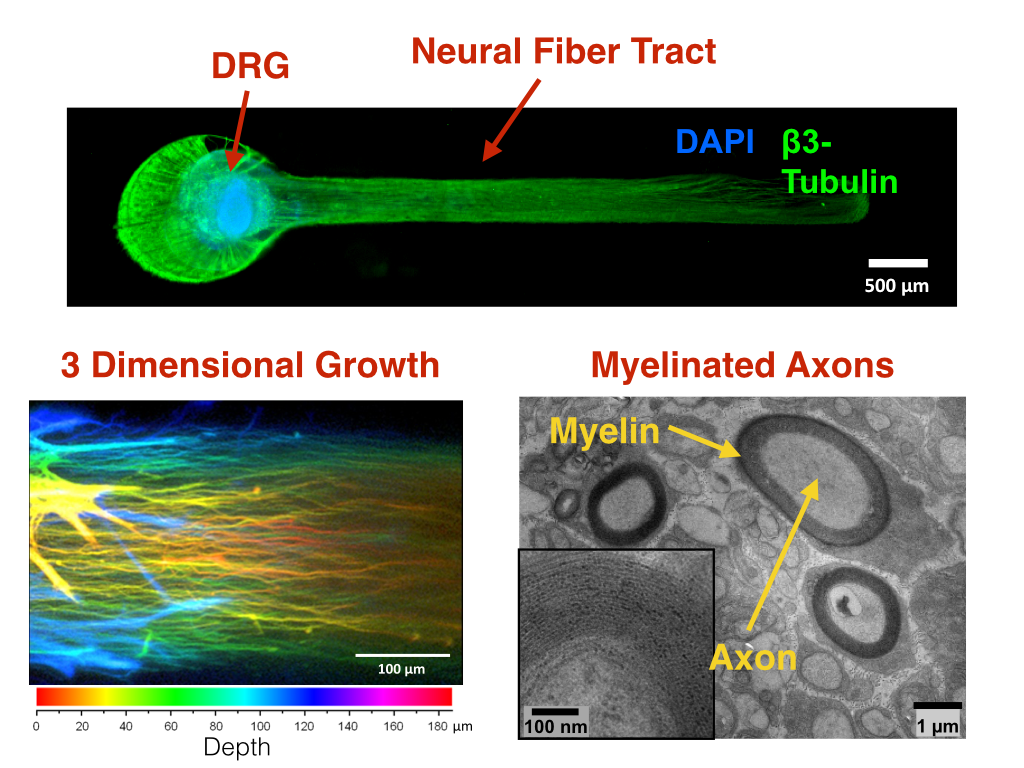
Figure 1: Microengineered myelinated neural fiber tract 3D architecture resembling native peripheral nerve anatomy.
Materials and Methods: Dorsal root ganglia (DRG) from embryonic day 15 rats were grown over a period of 4 weeks in a dual hydrogel system consisting of a methacrylated heparin gel in a polyethylene glycol micro-mold. The dual hydrogel system served to constrain the axon growth to be polarized with high density, fasciculation and myelination. The DRG’s were subject to a three-stage media regimen including ascorbic acid (AA) in the final stage to induce Schwann Cell myelination. The myelinated cultures were subjected to 60mM concentrations of the D-glucose in media over a period of 48 hours to simulate diabetic neuropathy. The control cultures were exposed to a 25mM D-glucose concentration. Electrophysiological field recordings were taken with and without the exposure of high glucose, and changes in the compound action potentials in the field recording were observed and compared to the control.
Results and Discussion: Compound action potentials show little or no synaptic input, increased delay and decreased amplitude with higher glucose concentration exposure as shown in Figure 2. The higher glucose concentration is thought to trigger the sorbitol pathway and induce oxidative stress in the neurons of the DRG, leading to apoptosis and axonal degeneration.
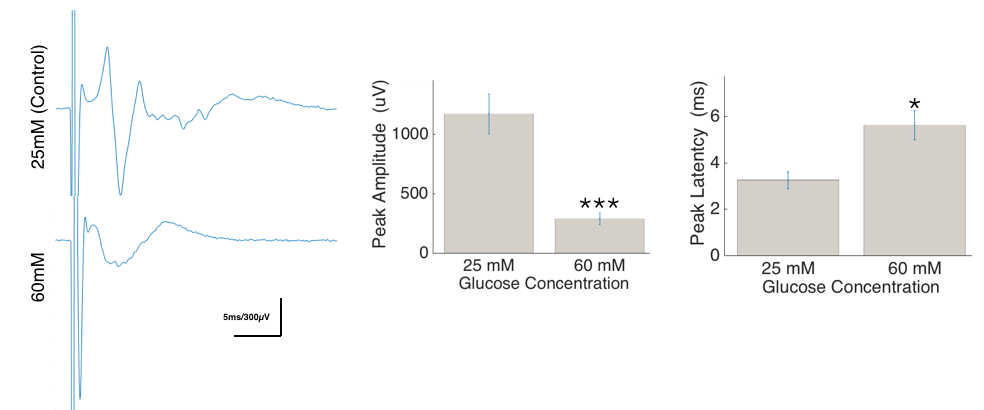
Figure 2: Average traces representing compound action potentials recorded with and without high glucose treatment. The change in amplitude and latency are also quantified as bar plots. (n=10, * = p<0.05 and ***=p<0.001)
Conclusion: Microengineered myelinated neural fiber tracts have morphology, physiology and, toxicity response analogous to a peripheral nerve. The platform could be used as an in-vitro model for neuropathy studies and drug development.
Funding provided by Tulane University
References:
[1] Gabbay, K. H., Merola, L. O. & Field, R. A. “Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes,” Science 151, 209–210 (1966).
[2] Renee M. Huval a, Oliver H. Miller b, J. Lowry Curley a, Yuwei Fan a, Benjamin J. Hall bc and Michael J. Moore."Microengineered peripheral nerve-on-a-chip for preclinical physiological testing." Lab Chip, 2015, 15, 2221-2232