Fractal, multifractal, and lacunarity analysis of microglia in tissue engineering
- Centre for Research in Complex Systems, School of Community Health, Charles Sturt University, Albury, NSW, Australia
Introduction
Tissue engineering is currently one of the most exciting fields in biology (Grayson et al., 2009). Fractal analysis is equally exciting (Di Ieva et al., 2013), as is the study of microglia, the brain’s immuno-inflammatory cell, recently shown to be of considerably more importance than previously imagined in both healthy and diseased brain (Tremblay et al., 2011). Each of these fields is developing at a pace far outstripping our capacity to integrate and translate the information gained into clinical use (Karperien et al., 2008b, 2013; Jelinek et al., 2011, 2013), and the excitement more than trebles where these fields intersect. Three elements of fractal analysis – monofractal, multifractal, and lacunarity analysis – applied to microglia may contribute significantly to the next steps forward in engineered tissues and 3D models in neuroscience.
Fractal Analysis and Lacunarity
To define “fractal analysis” would take a volume, but for this commentary, it is sufficient to understand that fractal analysis in biology assesses the scaling inherent in biological forms or events, and turns out a statistical index of complexity having no units called the “fractal dimension” (DF). This number measures not length, width, height, or density, but scale-invariant detail. For a pattern to have fractal scale-invariant detail means that the pattern repeats itself infinitely as one inspects it at closer and closer resolution (magnifies it), where that detail is not trivial. To elaborate, as one magnifies a simple line, it infinitely repeats itself quite trivially as a simple line, but as one magnifies a fractal line, one finds it never resolves into straight pieces but rather each magnified segment repeats the initial fractal pattern infinitely. A DF measures this infinite scaling, quantifying complex patterns without rendering meaningless the relative numbers of large and small measurements within them. Without getting too technical, fractal analysis of a simple line yields a DF of 1.00, and the higher the “complexity,” the higher the DF (Mandelbrot, 1983; Takayasu, 1990). Building on this so-called monofractal analysis, multifractal analysis, to summarize, is a way of finding for a single pattern a spectrum of DFs, owing to a pattern having characteristically multiple degrees of scaling, such as could be imagined for a cascading fractal phenomenon (Jestczemski and Sernetz, 1996; Falconer, 2014).
The word “lacuna” is derived from the word for lake, and refers to a gap or pool. In fractal analysis, lacunarity translates to measures of gappiness or “visual texture,” such as might be seen in the patchiness of forests, for instance (Plotnick et al., 1993). It has been defined as the degree of inhomogeneity and translational and rotational invariance in an image (Plotnick et al., 1993; Smith et al., 1996), where low lacunarity implies homogeneity and that rotating the image will not change it significantly. Thus, an image having mostly similarly sized gaps and little rotational variance would be expected to have low lacunarity, and one with much heterogeneity, many differently sized gaps, and notable rotational variance, would be expected to have high lacunarity (Karperien et al., 2011a). Lacunarity is frequently assessed during fractal analysis because the data on which it is based are easily collected by the same methods. The details and calculations behind fractal analysis are beyond the scope of this commentary but user-friendly, freely available software for biologists (Karperien, 2001, 2013) and in-depth explanations are available elsewhere (Smith et al., 1996).
Microglia
Microglia are of considerable interest to the tissue engineer interested in the central nervous system (CNS). These are tiny immuno-inflammatory cells that are very abundant in and wield considerable power in the brain and spinal cord of humans as well as many other species (Dowding and Scholes, 1993; Sheffield and Berman, 1998; Bernhardi and Nicholls, 1999; Sierra et al., 2014a). They are considered structural in some senses, and are indeed immune cells, yet traffic through the CNS, and are not grossly separated from their surroundings in the way that the meninges can be peeled from the brain or lymph nodes are segregated from surrounding tissue, for instance. Similar in number to neurons but much smaller in size, microglia in living organisms are usually found as individual cells physically integrated within the tangled mesh of cells that is the CNS (Lawson et al., 1990; Rezaie and Male, 1999; Billiards et al., 2006; Inoue, 2006; Stoll et al., 2006; Leung et al., 2008; Morgan et al., 2012; Zhao et al., 2012; Hinwood et al., 2013).
They play key roles in immature, developing nervous tissue, and in adult tissue, they ensure normal goings on but also police, protect, repair, and remodel neurons, including by removing cell parts and debris (Sierra et al., 2014a,b). They are meaningfully involved in virtually everything that goes on in the brain, from mediating behavioral effects of emotional stress (Hinwood et al., 2013) to autism (Maezawa et al., 2011; Morgan et al., 2012) to cleaning up after a stroke (Vinet et al., 2012). The scientific community has show-cased them using time-lapse photography and in vivo thin-skull visualization, revealing how they move within their space, by furling, unfurling, and waving their processes about, and throughout their space, migrating and phagocytosing (Nimmerjahn et al., 2005; Tremblay et al., 2011). Marvelously, they have no single form, rather, they exist along a highly disparate continuum of forms, shape-shifting to meet the most immediate challenge to the neurons they support, morphing back and forth as required (Karperien et al., 2013). Indeed, their function is usually inferred largely from their form, albeit generally backed up with biochemical and other data (Streit and Kreutzberg, 1987; Kreutzberg, 1995; Banati et al., 1999; Orlowski et al., 2003; Sheets et al., 2013).
Measuring Microglia with Fractal Analysis
What is perhaps most marvelous of all is that their morphology can be measured by their DF, as well as their lacunarity, and to some extent multifractal spectra (Soltys et al., 2001; Jelinek et al., 2008, 2011; Karperien et al., 2008c, 2011b, 2013). Finding this was a relief to the beleaguered microgliologist, because microglial morphology is not easily quantifiable by traditional measures despite that microglial function is so well-correlated with that morphology. Basically, while microglia change shape back and forth from highly ramified usually radially branched structures to plump and rounded blobs, their DFs range from higher to lower values corresponding to the spectrum of morphological change (see Figure 1) (Karperien et al., 2013). Results of in silico modeling studies agree with these general conclusions from studies of actual cells, showing microglia can be successfully modeled using sets of increasingly complex fractal branching parameters (Jelinek et al., 2002; Jelinek and Karperien, 2008).
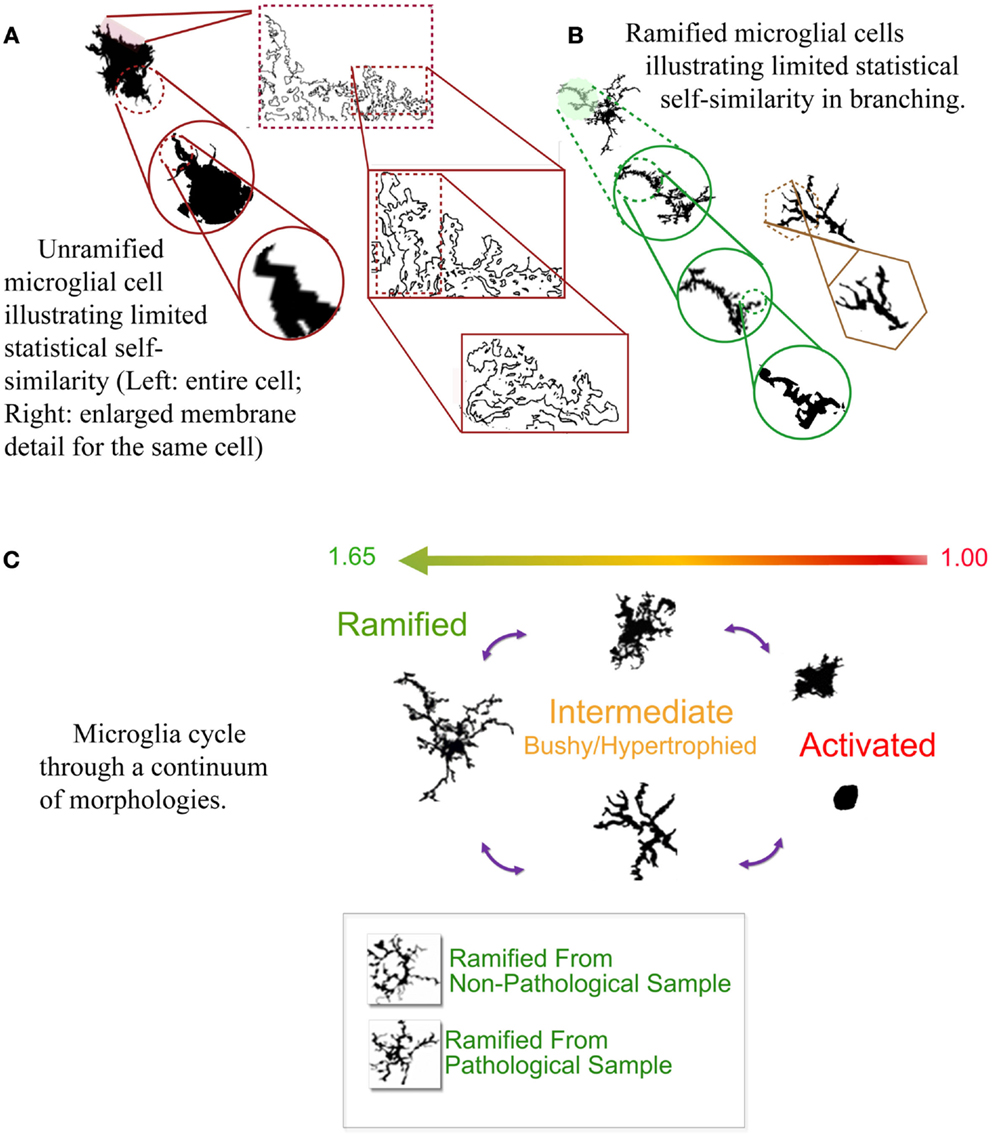
Figure 1. (A,B) Self-similarity in two typical microglial morphologies. (C) The cycle of microglial morphology. Microglia adopt morphologies along a cycle that corresponds to their box-counting DF (shown ranging from 1.00 to 1.65 in the figure). Ramified morphologies are more complex, and the most activated, rounded forms least complex. The bottom of (C) shows two cells from pathological vs. non-pathological tissue that were visually indistinguishable but objectively distinguishable by the box-counting DF. Author’s figures adapted from Karperien et al. (2013).
The practical value of fractal analysis surpasses classifying individual cells and verifying models. The DF has been used to analyze overall status in pathological conditions and aging (Jelinek et al., 2008; Karperien et al., 2008a,c). Data from biological and in silico cells (Jelinek et al., 2002) suggest the significance of multifractal scaling in particular is that it identifies microglia in temporarily hyper-ramified transitional states between ramified and intermediately activated forms.
Lacunarity, like multifractal spectra, also complements the DF. Lacunarity and DFs have been shown to be correlated in some research, but not by all methods of fractal analysis. For microglia, the box-counting DF and lacunarity both generally decrease as cells cycle toward a more activated state, then increase as they return to a ramified state (Jelinek et al., 2008), but this is not strictly the case and the exceptions are meaningful. It has been established using box-counting fractal analysis methods that some patterns indistinguishable by their DFs are distinguishable by their lacunarity, or vice versa, and such is the case for microglia (Karperien et al., 2011a, 2013). In silico modeling of microglia has shown that although the DF is generally more sensitive using whole cells, lacunarity is more sensitive to changes in particular features such as soma size relative to process length (Karperien et al., 2011a, 2013). Lacunarity has also been demonstrated to better identify microglia than does the DF in certain situations (e.g., elderly human cortex but not tumor) (Soltys et al., 2005; Karperien et al., 2011a).
Conclusion
To sum up, our point here is twofold: first, to let the tissue engineer modeling CNS know that he or she needs to consider microglia, because despite that these cells are tiny and were once considered negligible for normal function, they are entirely engaged physically and physiologically within the CNS; and second, to ensure that he or she is aware that these cells are characterized by some degree of fractal scaling. When developing methods to restore and replace diseased tissue, the tissue engineer who does not consider these two factors may develop models that overlook or misrepresent events. In particular, there is a need to ensure that models, such as engineered tissues being used as 3D in vivo models and cell-culture models being used for things like pharmaceutical research, do not overlook ostensibly subtle features of microglial activity that are characterizable by fractal measures but not traditional measures, and may be very important (Leung et al., 2008; Katari et al., 2014). The work discussed here focused on individual cell changes, but such changes can be understood within broader notions of decreasing complexity with increasing pathology, perhaps attributable to decreasing ability to generate novel responses to deal with rapidly changing environments. At any rate, for engineering and modeling CNS, from cell-culture environments to tissue formation and function, microglia are tiny but critical components, and their fractal and multifractal features need to be considered.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Lucas Karperien, biomedical engineering student, Faculty of Engineering, University of Victoria, Canada, for helpful review of the manuscript.
References
Banati, R. B., Goerres, G. W., Myers, R., Gunn, R. N., Turkheimer, F. E., Kreutzberg, G. W., et al. (1999). [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology 53, 2199–2203. doi:10.1212/WNL.53.9.2199
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bernhardi, R. V., and Nicholls, J. G. (1999). Transformation of leech microglial cell morphology and properties following co-culture with injured central nervous system tissue. J. Exp. Biol. 202(Pt 6), 723–728.
Billiards, S. S., Haynes, R. L., Folkerth, R. D., Trachtenberg, F. L., Liu, L. G., Volpe, J. J., et al. (2006). Development of microglia in the cerebral white matter of the human fetus and infant. J. Comp. Neurol. 497, 199–208. doi:10.1002/cne.20991
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Di Ieva, A., Grizzi, F., Jelinek, H., Pellionisz, A. J., and Losa, G. A. (2013). Fractals in the neurosciences, part I general principles and basic neurosciences. Neuroscientist 20, 403–417. doi:10.1177/1073858413513927
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dowding, A. J., and Scholes, J. (1993). Lymphocytes and macrophages outnumber oligodendroglia in normal fish spinal cord. Proc. Natl. Acad. Sci. U.S.A. 90, 10183–10187. doi:10.1073/pnas.90.21.10183
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Falconer, K. (2014). Fractal Geometry: Mathematical Foundations and Applications. Chichester: Wiley.
Grayson, W. L., Martens, T. P., Eng, G. M., Radisic, M., and Vunjak-Novakovic, G. (2009). Biomimetic approach to tissue engineering. Semin. Cell Dev. Biol. 20, 665–673. doi:10.1016/j.semcdb.2008.12.008
Hinwood, M., Tynan, R. J., Charnley, J. L., Beynon, S. B., Day, T. A., and Walker, F. R. (2013). Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb. Cortex 23, 1784–1797. doi:10.1093/cercor/bhs151
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Inoue, K. (2006). “ATP receptors of microglia involved in pain,” in Novartis Foundation Symposium, Vol. 276 (Chichester: Wiley), 263–272; discussion 273–281.
Jelinek, H., Karperien, A., Buchan, A., and Bossomaier, T. (2008). Differentiating grades of microglia activation with fractal analysis. Complex. Int. 12, 1–12.
Jelinek, H., Karperien, A., Cornforth, D., Cesar, R., and Leandro, J. (2002). “MicroMod: an L-systems approach to neuron modelling,” in Proceedings of the Sixth Australasia-Japan Joint Workshop on Intelligent and Evolutionary Systems, (Canberra, ACT: Australian National University), 156–163.
Jelinek, H. F., and Karperien, A. (2008). “Microglia modelling and analysis using L-systems grammar,” in BIOSTEC 2008 International Joint Conference on Biomedical Engineering Systems and Technologies, eds P. Encarnação and A. Veloso (Funchal: Institute for Systems and Technologies of Information, Control and Communication (INSTICC)), 289–294.
Jelinek, H. F., Karperien, A., and Milosevic, N. T. (2011). “Lacunarity analysis and classification of microglia in neuroscience,” in Proceedings of the 8th European Conference on Mathematical and Theoretical Biology, Cracow.
Jelinek, H. F., Milošević, N. T., Karperien, A., and Krstonošić, B. (2013). “Box-counting and multifractal analysis in neuronal and glial classification,” in Advances in Intelligent Control Systems and Computer Science, ed. I. Dumitrache (Berlin: Springer), 177–189.
Jestczemski, F., and Sernetz, M. (1996). Multifractal approach to inhomogeneous fractals. Physica A 223, 275–282. doi:10.1016/0378-4371(95)00365-7
Karperien, A. (2001). FracLac User’s Guide [Online]. Available at: http://rsbweb.nih.gov/ij/plugins/fraclac/FLHelp/Introduction.htm
Karperien, A., Ahammer, H., and Jelinek, H. F. (2013). Quantitating the subtleties of microglial morphology with fractal analysis. Front. Cell. Neurosci. 7:3. doi:10.3389/fncel.2013.00003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Karperien, A., Jelinek, H., and Milosevic, N. (2011a). “Reviewing lacunarity analysis and classification of microglia in neuroscience,” in 8th European Conference on Mathematical and Theoretical Biology (Cracow: European Society for Mathematical and Theoretical Biology (ESMTB)).
Karperien, A., Jelinek, H. F., and Milošević, N. T. (2011b). “Multifractals: a review with an application in neuroscience,” in CSCS18-18th International Conference on Control Systems and Computer Science: Fifth Symposium on Interdisciplinary Approaches in Fractal Analysis IAFA 1.5 (Cracow), 888–893.
Karperien, A., Jelinek, H. F., and Bossomaier, T. (2008a). Fractal analysis quantitates overt and subtle effects of naloxone and lipopolysaccharide on cultured rat microglia. Complex. Int. 12, Paper ID: msid12. Available at: http://www.complexity.org.au/ci/vol12/msid12/file.pdf
Karperien, A., Jelinek, H. F., Leandro, J. J., Soares, J. V., Cesar, R. M., and Luckie, A. (2008b). Automated detection of proliferative retinopathy in clinical practice. Clin. Ophthalmol. 2, 109–122. doi:10.2147/OPTH.S1579
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Karperien, A. L., Jelinek, H. F., and Buchan, A. M. (2008c). Box-counting analysis of microglia form in schizophrenia, Alzheimer’s disease and affective disorder. Fractals 16, 103. doi:10.1142/S0218348X08003880
Katari, R., Peloso, A., and Orlando, G. (2014). Tissue engineering and regenerative medicine: semantic considerations for an evolving paradigm. Front. Bioeng. Biotechnol. 2:57 doi:10.3389/fbioe.2014.00057
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kreutzberg, G. W. (1995). Microglia, the first line of defence in brain pathologies. Arzneimittelforschung 45, 357–360.
Lawson, L. J., Perry, V. H., Dri, P., and Gordon, S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170. doi:10.1016/0306-4522(90)90229-W
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leung, B. K., Biran, R., Underwood, C. J., and Tresco, P. A. (2008). Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials 29, 3289–3297. doi:10.1016/j.biomaterials.2008.03.045
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maezawa, I., Calafiore, M., Wulff, H., and Jin, L. W. (2011). Does microglial dysfunction play a role in autism and Rett syndrome? Neuron Glia Biol. 7, 85–97. doi:10.1017/S1740925X1200004X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Morgan, J. T., Chana, G., Abramson, I., Semendeferi, K., Courchesne, E., and Everall, I. P. (2012). Abnormal microglial-neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res. 1456, 72–81. doi:10.1016/j.brainres.2012.03.036
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nimmerjahn, A., Kirchhoff, F., and Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. doi:10.1126/science.1110647
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Orlowski, D., Soltys, Z., and Janeczko, K. (2003). Morphological development of microglia in the postnatal rat brain. A quantitative study. Int. J. Dev. Neurosci. 21, 445–450. doi:10.1016/j.ijdevneu.2003.09.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Plotnick, R., Gardner, R., and O’neill, R. (1993). Lacunarity indices as measures of landscape texture. Landsc. Ecol. 8, 201–211. doi:10.1007/BF00125351
Rezaie, P., and Male, D. (1999). Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc. Res. Tech. 45, 359–382. doi:10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sheets, K. G., Jun, B., Zhou, Y., Zhu, M., Petasis, N. A., Gordon, W. C., et al. (2013). Microglial ramification and redistribution concomitant with the attenuation of choroidal neovascularization by neuroprotectin D1. Mol. Vis. 19, 1747–1759.
Sheffield, L. G., and Berman, N. E. (1998). Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol. Aging 19, 47–55. doi:10.1016/S0197-4580(97)00168-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sierra, A., Beccari, S., Diaz-Aparicio, I., Encinas, J. M., Comeau, S., and Tremblay, M. E. (2014a). Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014, 610343. doi:10.1155/2014/610343
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sierra, A., Tremblay, M. E., and Wake, H. (2014b). Never-resting microglia: physiological roles in the healthy brain and pathological implications. Front. Cell. Neurosci. 8:240. doi:10.3389/fncel.2014.00240
Smith, T. G. Jr., Lange, G. D., and Marks, W. B. (1996). Fractal methods and results in cellular morphology – dimensions, lacunarity and multifractals. J. Neurosci. Methods 69, 123–136. doi:10.1016/S0165-0270(96)00080-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Soltys, Z., Orzylowska-Sliwinska, O., Zaremba, M., Orlowski, D., Piechota, M., Fiedorowicz, A., et al. (2005). Quantitative morphological study of microglial cells in the ischemic rat brain using principal component analysis. J. Neurosci. Methods 146, 50–60. doi:10.1016/j.jneumeth.2005.01.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Soltys, Z., Ziaja, M., Pawlinski, R., Setkowicz, Z., and Janeczko, K. (2001). Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J. Neurosci. Res. 63, 90–97. doi:10.1002/1097-4547(20010101)63:1<90::AID-JNR11>3.0.CO;2-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stoll, M., Capper, D., Dietz, K., Warth, A., Schleich, A., Schlaszus, H., et al. (2006). Differential microglial regulation in the human spinal cord under normal and pathological conditions. Neuropathol. Appl. Neurobiol. 32, 650–661. doi:10.1111/j.1365-2990.2006.00774.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Streit, W. J., and Kreutzberg, G. W. (1987). Lectin binding by resting and reactive microglia. J. Neurocytol. 16, 249–260. doi:10.1007/BF01795308
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tremblay, M. E., Stevens, B., Sierra, A., Wake, H., Bessis, A., and Nimmerjahn, A. (2011). The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069. doi:10.1523/JNEUROSCI.4158-11.2011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vinet, J., Weering, H. R., Heinrich, A., Kalin, R. E., Wegner, A., Brouwer, N., et al. (2012). Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J. Neuroinflammation 9, 27. doi:10.1186/1742-2094-9-27
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhao, Y. N., Wang, F., Fan, Y. X., Ping, G. F., Yang, J. Y., and Wu, C. F. (2012). Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav. Brain Res. 236C, 270–282. doi:10.1016/j.bbr.2012.08.052
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: microglia, fractal analysis, multifractal, lacunarity, tissue engineering
Citation: Karperien AL and Jelinek HF (2015) Fractal, multifractal, and lacunarity analysis of microglia in tissue engineering. Front. Bioeng. Biotechnol. 3:51. doi: 10.3389/fbioe.2015.00051
Received: 12 January 2015; Paper pending published: 27 February 2015;
Accepted: 27 March 2015; Published online: 14 April 2015.
Edited by:
Andres Diaz Lantada, Universidad Politécnica de Madrid, SpainReviewed by:
Antonio Di Ieva, University of Toronto, CanadaCopyright: © 2015 Karperien and Jelinek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: hjelinek@csu.edu.au
 Audrey L. Karperien
Audrey L. Karperien Herbert F. Jelinek
Herbert F. Jelinek