Recent developments in synthesis of xLi2MnO3 · (1 − x)LiMO2 (M = Ni, Co, Mn) cathode powders for high-energy lithium rechargeable batteries
- Department of Chemical Engineering and Waterloo Institute for Nanotechnology, University of Waterloo, Waterloo, ON, Canada
Lithium-rich layered powders, Li2MnO3-stabilized LiMO2 (M = Ni, Co, Mn), are attractive cathode candidates for the next generations of high-energy lithium-ion batteries. However, most of the state-of-the-art preparation procedures are complicated and require multiple energy-intensive reaction steps. Thus, elucidating a low-cost synthetic protocol is important for the application of these materials in future lithium-ion batteries. Recent developments in the synthesis procedures of lithium-rich layered powders are discussed and future directions are pointed out in this review.
Introduction
From its first commercial introduction by Sony in 1991, the LiCoO2 cathode has been widely used in portable electronics due to its excellent rate capability, cyclability, and high-tap density (Ying et al., 2004; He et al., 2006). Although the theoretical specific capacity of LiCoO2 electrodes is 273 mAh g−1, the practical specific capacity is ~140 mAh g−1 due to high-surface reactivity and instability of the delithiated Li1−xCoO2 (x > 0.5) (Thackeray et al., 2007; Nagai et al., 2011). Cobalt, however, is relatively expensive and somewhat toxic, thus, there is a great need to find new cathode materials with superior capacity, energy, safety, and lower cost.
Argonne National Laboratory initiated research to develop a family of Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) cathodes for lithium-ion batteries (Kim et al., 2002, 2004; Thackeray et al., 2005). These electrode materials have similar layered structure to LiCoO2, but possess superior stability, which allows the extraction of higher quantities of lithium ions during charge over a wider operating potential range, such as 2.0–4.6 V. As a result, practical specific discharge capacity of more than 200 mAh g−1 can be achieved at an average discharge potential of ~3.6 V vs. Li+/Li°. In addition, thermal stability of these electrodes is significantly greater than that of LiCoO2. Furthermore, less cobalt usage leads to lower material cost and environmental hazard.
There are excellent reviews (Thackeray et al., 2005, 2007; He et al., 2012; Yu and Zhou, 2013) describing molecular structure, thermal stability, electrochemistry, cycling stability, and other physical as well as electrochemical properties of xLi2MnO3·(1 − x)LiMO2 for cathode application. However, there is no review, which explains the synthetic procedures of these cathode materials. Because the synthesis method greatly determines the material properties and its commercial feasibility, a systematic review of synthesis procedures of xLi2MnO3·(1 − x)LiMO2 powders is crucial, especially for researchers who are entering high-energy lithium-ion battery research. In this review, we discuss many synthesis pathways of xLi2MnO3·(1 − x)LiMO2 powders for cathode application in high-energy lithium-ion batteries.
Solid-State Synthesis
Solid-state synthesis is a conventional method for the preparation of electrochemically active materials for lithium-ion batteries. It is considered as a simple and scalable method. However, several choices of precursors, temperature profiles, cooling modes … should be identified and optimized. The solid-state process includes several successive steps such as mixing, milling, grinding, pelletizing, annealing, and quenching. In many cases, ball milling devices may be used for the purpose of fast and effective mixing and grinding. However, simple grinding and mixing by mortar and pestle are effective and widely accepted. Since reactions are controlled by solid-state diffusion, time, and energy consumption are important factors. For preparation of xLi2MnO3·(1 − x)LiMO2 (M = Ni, Co, Mn), the precursors consist of transition metal salts (acetates of Ni2+, Co2+, Mn2+) (Wang et al., 2012; Yu and Zhou, 2012; Yu et al., 2012a,b, 2013a), manganese–nickel–cobalt hydroxides (Kim et al., 2002, 2004; Johnson et al., 2007; Li et al., 2011; Xu et al., 2011; Zhang et al., 2012), or carbonates (Deng et al., 2010; Croy et al., 2011; Koenig et al., 2011; Wang et al., 2011), and a lithium-containing compound (usually lithium hydroxide). The stoichiometric mixture is initially decomposed at 480–600°C for 3–15 h, then pelletized and calcined at 850–1000°C for 10–24 h in air. The sample can be rapidly quenched (in air or liquid nitrogen) (Kim et al., 2002; Ito et al., 2008; Wu and Manthiram, 2009; Madhu et al., 2010; Li et al., 2011; Zhang et al., 2012) or furnace cooled (Wang et al., 2012).
Solid-State Reaction in Combination with Co-Precipitation
Most xLi2MnO3·(1 − x)LiMO2 samples are prepared by a combination of co-precipitation and solid-state reactions (or mixed hydroxide method), following the pioneer work at Argonne National Laboratory (Kim et al., 2002, 2004). Scanning electron microscopy images of a typical product is shown in Figure 1 (Li et al., 2011), which represent the non-uniformity of the solid-state synthetic products. The manganese–nickel–cobalt hydroxide precursor is prepared by co-precipitation of transition metal aqueous solutions (nitrate or acetate of Ni2+, Co2+, Mn2+) in a basic aqueous solution (sodium, potassium, or lithium hydroxide) (Kim et al., 2002; Kang et al., 2006; Johnson et al., 2007, 2008; Park et al., 2007a; Wu and Manthiram, 2009; Li et al., 2011; Xu et al., 2011). In order to prepare the hydroxide precursor with uniform particle size, polyvinylpyrrolidone (PVP) and ethylene glycol (EG) are used as dispersants (Xiang et al., 2013). PVP hinders the growth space of particles due to micelle formation. EG limits the growth rate of particles because of the high viscosity. Subsequently, the dried material is mixed with lithium hydroxide to produce the starting mixture for solid-state reactions.
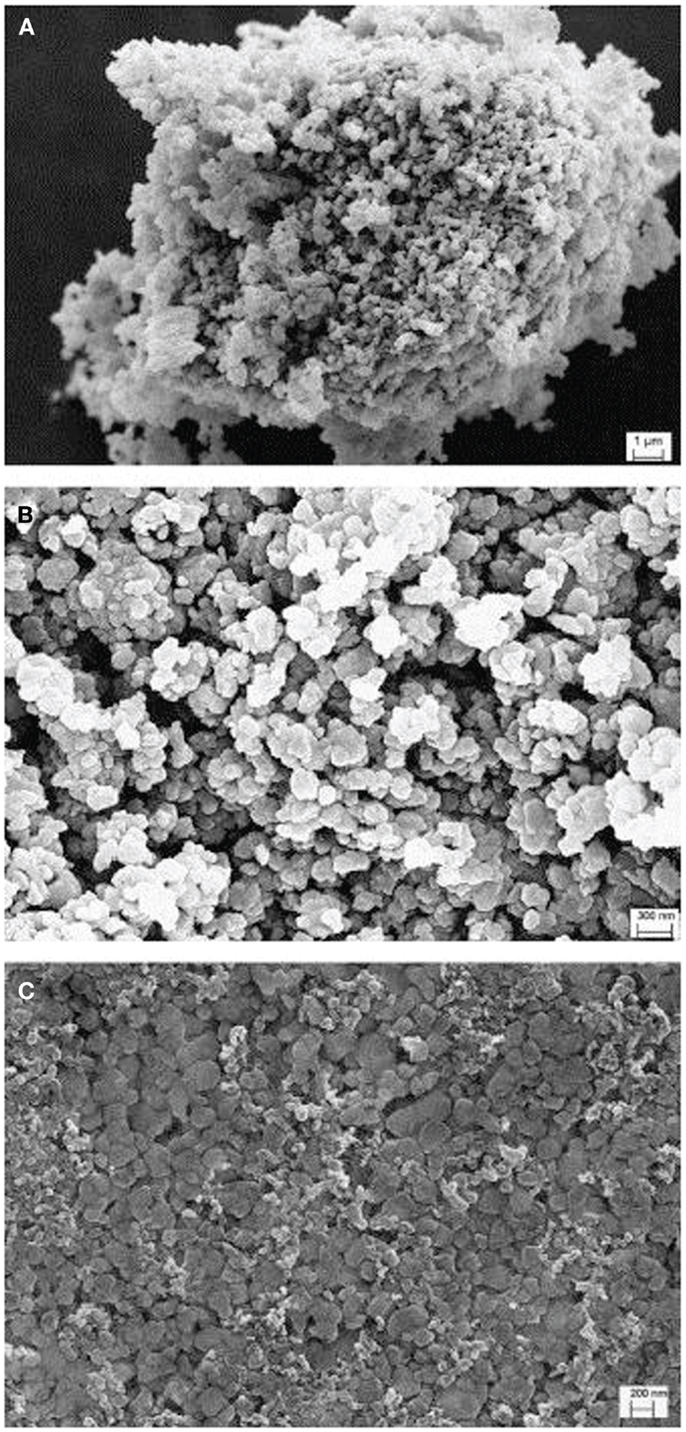
Figure 1. SEM pictures of Li[Li0.2Mn0.56Ni0.16Co0.08]O2 materials at different magnifications. (A) ×5k; (B,C) ×50k. Reproduced with permission from Li et al. (2011).
Deng et al. (2010) reported the preparation of Li(1+x)Ni0.25 Mn0.75O(2.25+x/2) (where x = 0.32–0.65), which contains the Li2MnO3 phase, by using all carbonate precursors. A spherical Ni0.25Mn0.75CO3 precursor is first prepared by co-precipitation, followed by solid-state reaction between that precursor and a stoichiometric amount of Li2CO3. Similarly, 0.5Li2MnO3 0.5LiCoO2 is synthesized by solid-state reaction between (Co0.5Mn0.5)CO3 and Li2CO3 (Croy et al., 2011). (Co0.5Mn0.5)CO3 is prepared beforehand by mixing a solution of cobalt and manganese sulfate with ammonium bicarbonate solution. In addition, NixMn1−xCO3 can be prepared from nickel and manganese sulfate, sodium carbonate, and ammonium hydroxide (Lee et al., 2006; Ito et al., 2008; Koenig et al., 2011; Wang et al., 2011). Another transition metal precursor is NiMnO3, which can be prepared by heating nickel manganese double hydroxides at 600°C for 4 h, then NiMnO3 is mixed with lithium hydroxide for the subsequent solid-state reactions (Ohzuku et al., 2011). Using a combination of co-precipitation and solid-state reaction techniques, higher homogeneity is achieved, thus, improved discharge capacity such as 270 mAh g−1 at 0.1 C in the voltage range of 2–4.6 V was reported (Lee et al., 2006). However, adding several steps to the conventional solid-state reaction leads to time and cost increases that may hinder scale-up and commercialization.
Solid-State Reaction
Pure solid-state preparation of xLi2MnO3·(1 − x)LiMO2 has been practised by Zhou’s group (AIST, Japan). By simple solid-state reactions of lithium hydroxide and transition metal acetates, cathode materials with sufficient high-specific capacity (~225 mAh g−1 at 0.1 C, 2–4.8 V) can be achieved (Yu et al., 2012b). (1 − x − y)LiNiO2·xLi2MnO3·yLiCoO2 samples can be synthesized by solid-state reactions of all acetate precursors (Madhu et al., 2010). The optimized sample exhibits a discharge capacity of 244 mAh g−1 at C/15 rate in the potential range of 2–4.6 V. Oxalic acid can be used as a precipitant in the precursor mixture in order to produce dry precursor and to obtain higher homogeneous distributions of transition metal ions (Wang et al., 2012).
Sol-Gel Method
The sol-gel method appears to be the another good choice for the preparation of xLi2MnO3·(1 − x)LiMO2 cathode materials. This method produces high purity and homogeneous products but is time consuming due to aging and drying. Stoichiometric amounts of lithium acetate, nickel acetate, cobalt acetate, and manganese acetate (or nitrate salts) are dissolved in distilled water (Wu et al., 2010; Jarvis et al., 2012; Jiang et al., 2013). An additional solution containing chelating agent such as citric acid (Wu et al., 2010), glycolic/tartaric acid (Kang et al., 2007), or citric acid/ethylenediaminetetraacetic acid (EDTA) (Jarvis et al., 2012) is added to the acetate solution under vigorous stirring to form a highly viscous gel. The solution pH is adjusted to ~7.5 using ammonium hydroxide. Water is evaporated at 70–80°C. The gel is decomposed at 450–500°C for 5–12 h and the solid product is ground after cooling. The resulting powders are pressed into pellets, heat treated at 800–900°C for 10–12 h. The final powder consists of nanosized primary particles, which are ~200–500 nm.
Combustion Method
Simple combustion is a popular method to prepare the lithium-rich layered cathode materials (Park et al., 2003, 2007b; Wu et al., 2004; Hong et al., 2005; Fu et al., 2014; Shen et al., 2014). In comparison with mixed hydroxide (solid-state reaction in combination with co-precipitation) and sol-gel method, combustion method is simpler and less expensive (Park et al., 2003). Precursors must contain at least one acetate salt, other precursors can be nitrate (Park et al., 2003, 2007b; Hong et al., 2005) or hydroxide (Wu et al., 2004). Stoichiometric amounts of Li, Ni, Mn, and Co sources are dissolved in distilled water under continuous stirring and evaporating at 80–100°C on a hot plate. The molar ratio of acetate to nitrate can be adjusted to 3:1 to keep the combustion condition stable (Hong et al., 2005). A viscous gel is obtained after evaporation of excess water. In case all precursors are acetates (Fu et al., 2014), mannitol can be added and pH is adjusted to 2 using concentrated nitric acid to assist the gel formation. The resulting gel is fired at 400°C for 30 min to 1 h in air. The obtaining ash-like powder is ground and heated at 500–700°C for 3 h in air, then reground and heated again at 900°C in air for 5–12 h. Finally, it is quenched to room temperature.
Other Synthesis Routes
Co-Precipitation
Similar to the sol-gel method, co-precipitation offers uniform particle size distribution as well as high-phase purity. However, few papers (Gim et al., 2012; Min et al., 2013; Yu et al., 2013b) described this method, which may be due to the complicated procedure and the difficulty in controlling the chemical composition. In a typical synthesis, transition metal acetate precursors and lithium hydroxide are dissolved separately in distilled water. The hydroxide co-precipitation of the transition metals is facilitated by slowly combining the aqueous lithium hydroxide solution with the solution containing transition metal precursors at room temperature over an extended period of time (e.g., 24 h) (Gim et al., 2012). The resulting precipitate is dried between 85 and 120°C, followed by a grinding period. The powders are then heated at 600°C for 3 h to decompose the organic compounds, then calcined at 900°C for 12 h to promote crystallization, and finally quenched (Min et al., 2013).
If lithium carbonate is used as the lithium source instead of lithium hydroxide (Yu et al., 2013a,b), the precursors are mixed in a basic solution containing ammonium heptamolybdate tetrahydrate. The precipitate is dried at 100°C and annealed at 950°C for 5 h in air.
Spray Pyrolysis
Spray pyrolysis (SP) is well known as a continuous and single-step preparation method for the synthesis of fine homogeneous and multicomponent powders. In comparison with the solid-state and sol-gel methods, SP is simpler and faster. In addition, the particle size distribution is typically narrow and controllable from the micrometer to submicrometer order, the purities of the products are high and the compositions of powders are easily controllable. Recently, the first paper reporting the preparation of xLi2MnO3·(1 − x)LiMO2 by SP (Zhang and Axelbaum, 2012) was published. It is expected that more reports employing this method of synthesis will be available since it requires time to master this relatively new and special technique.
In a typical synthesis, the precursor solution is prepared by dissolving stoichiometric amounts of lithium nitrate and transition metal nitrates in deionized water. An air-assisted nebulizer aerosolizes the precursor solution to produce fine micrometer droplets. The droplets are transferred into a tubular furnace by a constant flow of air. Temperature of the furnace is held at 700°C to ensure full decomposition of the nitrate precursors. The powders are collected by a filter, and then annealed at 700–800°C for 2 h.
Electrospinning
This method was adapted by Hosono et al. (2013) to prepare hollow nanowires of 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2. An aqueous solution containing lithium acetate, transition metal acetate precursors, and polyvinyl alcohol are dissolved and then aged at 90°C for 1 h in an aqueous solution containing methanol and acid acetic. The precursor solution is loaded on a syringe, which connects to a metal needle. Under a voltage of 20 kV across the aluminum foil collector and the metal needle, polymer wires containing the metal salts can be obtained. Then, the desired material with wire structure could be achieved by heat treatment at 800°C in air. This method may be good for laboratory experiments to produce one-dimensional materials, but may require sophisticated high-voltage instruments for scale-up purposes.
Reactive Mechanochemical Synthesis
The previously described solid-state method often delivers products with large particle sizes (up to micrometer) with broad distribution, uncontrollable particle growth, and agglomeration. To overcome this problem, reactive mechanochemical synthesis is included in the synthetic procedure. This method is effective to prepare composite with a more uniform distribution of precursor components. There are several parameters (e.g., mass of balls/sample, rotation speed, pressure, temperature, the presence of wetting agents …) that can be controlled to deliver effective synthesis.
Mechanochemical process was utilized by Kim et al. (2012) to prepare the xLi2MnO3·(1 − x)LiMO2 (M = Mn, Ni, Co). Li2MnO3 and LiMO2 (M = Ni, Mn, Co) can be incorporated together with controlled molar ratio thanks to the mechanochemical process (Figure 2). This incorporation of the two phases is apparently important because LiMO2 can be stabilized and Li2MnO3 is the source of excess lithium.
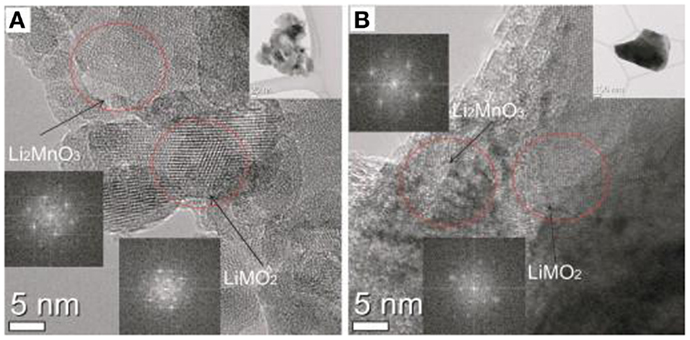
Figure 2. HRTEM images: (A) 0.5Li2MnO3·0.5LiMO2 (B) heat treated 0.5Li2MnO3·0.5LiMO2. Reproduced with permission of Kim et al. (2012).
In a typical synthesis, precursors (Li2MnO3 and LiNi0.5Co0.2 Mn0.3O2) and acetone are well mixed by using a planetary mill running at 350 rpm for 3 h. The composites are then heated at 1000°C for 10 h. Heat treated secondary particles are spherical. The performance of batteries prepared from this cathode material (discharge capacity is ~200 mAh g−1) is lower than those prepared by the solid-state reaction. The reasons are not yet clear, but it may be due to the lack of the pelletization step, and the use of the stable precursors.
Hydrothermal Method
Hydrothermal synthesis is performed at relatively low temperatures, thus, it is a low cost and energy-saving method to prepare fine particles. Lee et al. (2008) reported the preparation of layered Li0.88[Li0.18Co0.33Mn0.49]O2 nanowires via hydrothermal processing at 200°C. As prepared K0.3MnO2 is dispersed in a solution of Co(NO3)2 in distilled water, then kept in an autoclave at 200°C for 5 days, and washed thoroughly with water to remove residues and dissolved potassium ions. The obtained powders are Co0.4Mn0.6O2 nanowires. Finally, Co0.4Mn0.6O2 nanowires are mixed with LiNO3·H2O (wt/wt ratio is 4:1) in 100 ml of distilled water, and maintained in an autoclave at 200°C for 2 days. As-prepared powders are rinsed with water, and vacuum dried at 120°C. The materials exhibit excellent rate capability (220 mAh g−1 at 1 C charge and 15 C discharge between 2 and 4.8 V), but poor cyclability (capacity retention of 92% after 50 cycles at 1 C) due to the high-specific areas of the nanowires, which reduce the diffusion path (increases the rate capacity) and increase the electrode/electrolyte contact area (accelerates side reactions, especially at high-charge potentials). In addition, the procedure is complicated and time consuming.
Wei et al. (2013) proposed a simpler hydrothermal route for preparation of Li[Li0.2Co0.4Mn0.4]O2. A suspension containing stoichiometric amounts of lithium, manganese, cobalt acetates is prepared, with oxalic acid as a precipitating agent and acetic acid as an additive. This suspension is heated in a Teflon container at 150°C for 3 h, then dried, pelletized, preheated at 450–500°C, and finally calcined at 750°C in air. The cathode material exhibits relatively low-specific discharge capacity of 180 mAh g−1 between 2 and 4.6 V vs. Li+/Li° at 100 mA g−1. Moreover, high-temperature heat treatment is another disadvantage of this preparation route.
Surface Modifications
Surface of high-energy materials can be modified with Al2O3, CeO2, ZnO2, or ZnO (3 wt% in the final product) (Wu and Manthiram, 2009), TiO2 (Zheng et al., 2008), FePO4 (Wang et al., 2013), or treated with 0.1 M HNO3 (Kang et al., 2006). The surface modified cathodes with oxides (Wu and Manthiram, 2009) or FePO4 (Wang et al., 2013) show higher first coulombic efficiency, discharge capacity, and capacity retention than the unmodified samples due to the retention of higher number of oxide ion vacancies in the lattice after the first charge (Wu and Manthiram, 2009; Wang et al., 2013). In addition, it was reported that TiO2-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 has enhanced thermal stability and cyclability (Zheng et al., 2008). The authors proposed that coating layer prevents the direct contact between active material and electrolyte, thus, reduces the side reactions between them at high-charge voltage region. On the other hand, it is suggested that acid treatment eliminates the first cycle irreversible capacity loss (first coulombic efficiency is close to 100%) by chemical activation of the Li2MnO3 phase. However, the acid treatment significantly reduces the cyclability and rate capability because of the H+-exchange process and water entrapment inside the cathode powder (Kang et al., 2006). Immersion of the xLi2MnO3·(1 − x)LiMO2 powders in a Li–Ni–PO4 solution using sol-gel method, followed by a heat treatment period, leads to the formation of new materials with enhanced rate capability (Kang and Thackeray, 2009). Such treatment creates a stable protective Li–Ni–PO4 layer, which exhibits high-lithium-ion conductivity at high-working potentials (4.6 V vs. Li+/Li). Sun et al. (2012) reported that coating the high-energy cathode particles with AlF3 could improve their electrochemical performance, both rate capability and cyclability. It is proposed by the authors that the Li2MnO3 phase is selectively converted to the spinel structure by chemical leaching of Li from Li2MnO3 due to the presence of the AlF3 coating. Liu et al. (2010) introduced surface coating of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes by aluminum using a thermal evaporation technique. Aluminum coating increases the first discharge capacity and coulombic efficiency, improves the cyclability, and enhances the rate capability. The suppression of oxygen vacancy elimination at the end of first charge results in the increase in first discharge capacity, while the suppression of oxygen vacancy elimination and side reactions in the subsequent cycles leads to cyclability improvement. Rate capability enhancement can be explained by the increase in the surface conductivity. Zhao et al. (2011) reported that coating Li[Li0.2Ni0.2Mn0.6]O2 particles with manganese oxide (4 wt%) could significantly improve the cyclability and rate capability, although the reasons are not clear yet. Similar material was prepared by Wu et al. (2012), which exhibits lower charge transfer resistance in comparison with the uncoated one. In the study of Kang et al. (2005), Li[Li0.2Ni0.2Mn0.6]O2 is coated with amorphous Al(OH)3 created from the hydrolysis reaction of Al(C3H7O)3. The Al(OH)3 coated cathode has a lower charge transfer resistance than the uncoated one, thus, has better rate capability. Moreover, the Al(OH)3 coating also improves the thermal stability of the cathode powders.
Recently, Wu et al. (2014) proposed the coating of Li1.2 Mn0.6Ni0.2O2 by an ultrathin Li1+xMn2O4 layer. Polyvinylpyrrolidone is simply dispersed on the Li1.2Mn0.6Ni0.2O2 materials, and the mixture is heat treated at 750°C to form an ultrathin Li1+xMn2O4 membrane on the surface (Figure 3). This high-lithium conductive membrane promotes the lithium transportation between electrolyte and the layered active material, and stabilizes the layered bulk during high-voltage cycling. As a result, rate capability, cyclability, and thermal stability are all improved.
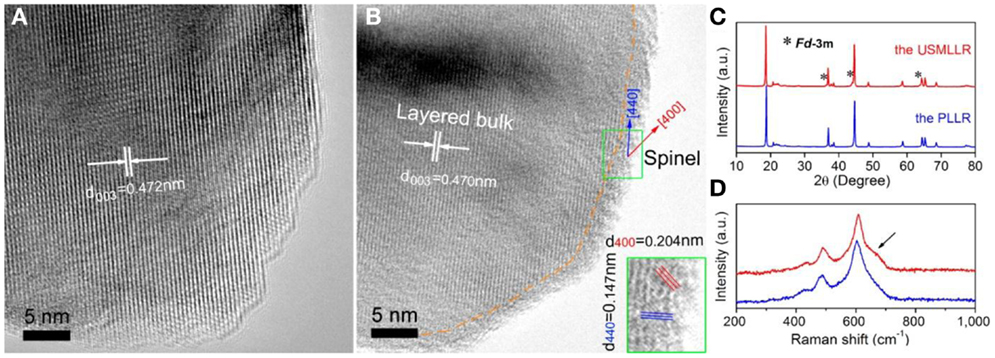
Figure 3. HRTEM images of the pristine layered lithium-rich materials (PLLR) sample (A) and the ultrathin spinel membrane encapsulated- layered lithium-rich cathode (USMLLR) sample (B). The inset is two times enlarged view in green rectangle of (B). XRD patterns (using Cu Kα radiation) (C) and Raman spectroscopies (D) of the PLLR sample and the USMLLR sample. Reproduced with permission from Wu et al. (2014).
Conclusion
Successful preparation of xLi2MnO3·(1 − x)LiMO2 cathode materials that exhibit a specific discharge capacity of 200 mAh g−1 or higher at 0.1 C rate between a potential range of 2–4.8 V vs. Li+/Li is crucial to the development of high-energy lithium-ion batteries. There may be a number of ways to achieve the challenge in research laboratories. From an engineering point of view, choosing a feasible preparation method for straightforward commercialization is the challenge since most of the current state-of-the-art methods require significant heating and long-processing times. Many research groups follow the initiation of researchers at the Argonne National Laboratory to prepare the cathode materials by the combination of co-precipitation and solid-state reactions. However, complicated procedures, long-heat treatment times and high-heat treatment temperatures are current drawbacks, which cloud the competitiveness of the high-energy cathode technology. It is believed that solid-state reaction, combustion, sol-gel, and co-precipitation methods will be further optimized and modified to deliver more promising battery performance (both specific capacity and cyclability), together with shortening the preparation times, reduction of heat treatment durations, and temperatures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by Positec, Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Foundation for Innovation (CFI), and the Canada Research Chairs (CRC) program. The authors would like to thank Dr. J. Byerley for assistance in editing the manuscript.
References
Croy, J. R., Balasubramanian, M., Kim, D., Kang, S.-H., and Thackeray, M. M. (2011). Designing high-capacity, lithium-ion cathodes using X-ray absorption spectroscopy. Chem. Mater. 23, 5415–5424. doi: 10.1021/cm2026703
Deng, H., Belharouak, I., Cook, R. E., Wu, H., Sun, Y.-K., and Amine, K. (2010). Nanostructured lithium nickel manganese oxides for lithium-ion batteries. J. Electrochem. Soc. 157, A447–A452. doi:10.1149/1.3308598
Fu, C., Li, G., Luo, D., Zheng, J., and Li, L. (2014). Gel-combustion synthesis of Li1.2Mn0.4Co0.4O2 composites with a high capacity and superior rate capability for lithium-ion batteries. J. Mater. Chem. A 2, 1471–1483. doi:10.1039/c3ta13920d
Gim, J., Song, J., Park, H., Kang, J., Kim, K., Mathew, V., et al. (2012). Synthesis and characterization of integrated layered nanocomposites for lithium ion batteries. Nanoscale Res. Lett. 7, 60–68. doi:10.1186/1556-276X-7-60
He, P., Wang, H., Qi, L., and Osaka, T. (2006). Synthetic optimization of spherical LiCoO2 and precursor via uniform-phase precipitation. J. Power Sources 158, 529–534. doi:10.1016/j.jpowsour.2005.08.044
He, P., Yu, H., Li, D., and Zhou, H. (2012). Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 22, 3680–3695. doi:10.1039/c2jm14305d
Hong, Y.-S., Park, Y. J., Ryu, K. S., Chang, S. H., and Shin, Y.-J. (2005). Structural and electrochemical properties of (1-x)Li[Ni0.20Li0.20Mn0.60]O2-xLi[Co0.50Li0.167Mn0.333]O2 for lithium secondary batteries. J. Power Sources 147, 214–219. doi:10.1016/j.jpowsour.2004.11.018
Hosono, E., Saito, T., Hoshino, J., Mizuno, Y., Okubo, M., Asakura, D., et al. (2013). Synthesis of LiNi0.5Mn1.5O4 and 0.5Li2MnO3-0.5LiNi1/3Co1/3Mn1/3O2 hollow nanowires by electrospinning. CrystEngComm 15, 2592–2597. doi:10.1039/c3ce26972h
Ito, A., Li, D., Ohsawa, Y., and Sato, Y. (2008). A new approach to improve the high-voltage cyclic performance of Li-rich layered cathode material by electrochemical pre-treatment. J. Power Sources 183, 344–346. doi:10.1016/j.jpowsour.2008.04.086
Jarvis, K. A., Deng, Z., Allard, L. F., Manthirama, A., and Ferreira, P. J. (2012). Understanding structural defects in lithium-rich layered oxide cathodes. J. Mater. Chem. 22, 11550–11555. doi:10.1039/c2jm30575e
Jiang, Y., Yang, Z., Luo, W., Hu, X., and Huang, Y. (2013). Hollow 0.3Li2MnO3 0.7LiNi0.5Mn0.5O2 microspheres as a high-performance cathode material for lithium-ion batteries. Phys. Chem. Chem. Phys. 15, 2954–2960. doi:10.1039/c2cp44394e
Johnson, C. S., Li, N., Lefief, C., and Thackeray, M. M. (2007). Anomalous capacity and cycling stability of xLi2MnO3·(1-x)LiMO2 electrodes (M = Mn, Ni, Co) in lithium batteries at 50°C. Electrochem. Commun. 9, 787–795. doi:10.1016/j.elecom.2006.11.006
Johnson, C. S., Li, N., Lefief, C., Vaughey, J. T., and Thackeray, M. M. (2008). Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3 (1-x)LiMn0.333Ni0.333Co0.333O2 (0 ≤ x ≤ 0.7). Chem. Mater. 20, 6095–6106. doi:10.1021/cm801245r
Kang, S.-H., Johnson, C. S., Vaughey, J. T., Amine, K., and Thackeray, M. M. (2006). The effects of acid treatment on the electrochemical properties of 0.5 Li2MnO3·0.5 LiNi0.44Co0.25Mn0.31O2 electrodes in lithium cells. J. Electrochem. Soc. 153, A1186–A1192. doi:10.1149/1.2194764
Kang, S.-H., Kempgens, P., Greenbaum, S., Kropf, A. J., Amine, K., and Thackeray, M. M. (2007). Interpreting the structural and electrochemical complexity of 0.5Li2MnO3 0.5LiMO2 electrodes for lithium batteries (M = Mn0.5-xNi0.5-xCo2x, 0 ≤ x ≤ 0.5). J. Mater. Chem. 17, 2069–2077. doi:10.1039/b618715c
Kang, S.-H., and Thackeray, M. M. (2009). Enhancing the rate capability of high capacity xLi2MnO3 (1-x)LiMO2 (M = Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment. Electrochem. Commun. 11, 748–751. doi:10.1016/j.elecom.2009.01.025
Kang, Y.-J., Kim, J.-H., Lee, S.-W., and Sun, Y.-K. (2005). The effect of Al(OH)3 coating on the Li[Li0.2Ni0.2Mn0.6]O2 cathode material for lithium secondary battery. Electrochim. Acta 50, 4784–4791. doi:10.1016/j.electacta.2005.02.032
Kim, J.-S., Johnson, C. S., Vaughey, J. T., and Thackeray, M. M. (2002). Layered xLiMO2·(1-x)Li2MO3 electrodes for lithium batteries: a study of 0.95LiMn0.5Ni0.5O2·0.05Li2TiO3. Electrochem. Commun. 4, 205–209. doi:10.1016/S1388-2481(02)00251-5
Kim, J.-S., Johnson, C. S., Vaughey, J. T., Thackeray, M. M., Hackney, S. A., Yoon, W., et al. (2004). Electrochemical and structural properties of xLi2M‘O3·(1-x)LiMn0.5Ni0.5O2 electrodes for lithium batteries (M‘ = Ti, Mn, Zr; 0 ≤ x ≤ 0.3). Chem. Mater. 16, 1996–2006. doi:10.1021/cm0306461
Kim, S., Kim, C., Noh, J.-K., Yu, S., Kim, S.-J., Chang, W., et al. (2012). Synthesis of layered-layered xLi2MnO3 (1-x)LiMO2 (M = Mn, Ni, Co) nanocomposite electrodes materials by mechanochemical process. J. Power Sources 220, 422–429. doi:10.1016/j.jpowsour.2012.07.135
Koenig, G. M., Belharouak, I., Deng, H., Sun, Y.-K., and Amine, K. (2011). Composition-tailored synthesis of gradient transition metal precursor particles for lithium-ion battery cathode materials. Chem. Mater. 23, 1954–1963. doi:10.1021/cm200058c
Lee, D.-K., Park, S.-H., Amine, K., Bang, H. J., Parakash, J., and Sun, Y.-K. (2006). High capacity Li[Li0.2Ni0.2Mn0.6]O2 cathode materials via a carbonate co-precipitation method. J. Power Sources 162, 1346–1350. doi:10.1016/j.jpowsour.2006.07.064
Lee, Y., Kim, M. G., and Cho, J. (2008). Layered Li0.88[Li0.18Co0.33Mn0.49]O2 nanowires for fast and high capacity Li-ion storage material. Nano Lett. 8, 957–961. doi:10.1021/nl0731466
Li, J., Klöpsch, R., Stan, M. C., Nowak, S., Kunze, M., Winter, M., et al. (2011). Synthesis and electrochemical performance of the high voltage cathode material Li[Li0.2Mn0.56Ni0.16Co0.08]O2 with improved rate capability. J. Power Sources 196, 4821–4825. doi:10.1016/j.jpowsour.2011.01.006
Liu, J., Reeja-Jayan, B., and Manthiram, A. (2010). Conductive surface modification with aluminum of high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes. J. Phys. Chem. C 114, 9528–9533. doi:10.1021/jp102050s
Madhu, C., Garrett, J., and Manivannan, V. (2010). Synthesis and characterization of oxide cathode materials of the system (1-x-y)LiNiO2·xLi2MnO3·yLiCoO2. Ionics 16, 591–602. doi:10.1007/s11581-010-0454-x
Min, J. W., Gim, J., Song, J., Ryu, W.-H., Lee, J.-W., Kim, Y.-I., et al. (2013). Simple, robust metal fluoride coating on layered Li1.23Ni0.13Co0.14Mn0.56O2 and its effects on enhanced electrochemical properties. Electrochim. Acta 100, 10–17. doi:10.1016/j.electacta.2013.03.085
Nagai, R., Kita, F., Yamada, M., and Katayama, H. (2011). Development of highly reliable high-capacity batteries for mobile devices and small- to medium-sized batteries for industrial applications. Hitachi Rev. 60, 28–32.
Ohzuku, T., Nagayama, M., Tsujia, K., and Ariyoshi, K. (2011). High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: toward rechargeable capacity more than 300 mA h g-1. J. Mater. Chem. 21, 10179–10188. doi:10.1039/c0jm04325g
Park, S.-H., Kang, S.-H., Johnson, C. S., Amine, K., and Thackeray, M. M. (2007a). Lithium-manganese-nickel-oxide electrodes with integrated layered-spinel structures for lithium batteries. Electrochem. Commun. 9, 262–268. doi:10.1002/adma.201104106
Park, Y. J., Kim, M. G., Hong, Y.-S., Wu, X., Ryu, K. S., and Chang, S. H. (2003). Electrochemical behavior of Li intercalation processes into a Li[NixLi(1/3-2x/3)Mn(2/3-x/3)]O2 cathode. Solid State Commun. 127, 509–514. doi:10.1016/S0038-1098(03)00432-0
Park, Y. J., Lee, J. W., Lee, Y.-G., Kim, K. M., Kang, M. G., and Lee, Y. (2007b). The structural and electrochemical properties of thermally aged Li[Co0.1Ni0.15Li0.2Mn0.55]O2 cathodes. Bull. Korean Chem. Soc. 28, 2226–2230. doi:10.5012/bkcs.2007.28.12.2226
Shen, C.-H., Wang, Q., Fu, F., Huang, L., Lin, Z., Shen, S.-Y., et al. (2014). Facile synthesis of the Li-rich layered oxide Li1.23Ni0.09Co0.12Mn0.56O2 with superior lithium storage performance and new insights into structural transformation of the layered oxide material during charge-discharge cycle: in situ XRD characterization. ACS Appl. Mater. Interfaces 6, 5516–5524. doi:10.1021/am405844b
Sun, Y.-K., Lee, M.-J., Yoon, C. S., Hassoun, J., Amine, K., and Scrosati, B. (2012). The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-ion batteries. Adv. Mater. 24, 1192–1196. doi:10.1002/adma.201104106
Thackeray, M., Kang, S.-H., Johnson, C. S., Vaughey, J. T., Benedek, R., and Hackney, S. A. (2007). Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112–3125. doi:10.1039/b702425h
Thackeray, M. M., Johnson, C. S., Vaughey, J. T., Li, N., and Hackney, S. A. (2005). Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 15, 2257–2267. doi:10.1039/b417616m
Wang, D., Belharouak, I., Koenig, G. M., Zhou, G., and Amine, K. (2011). Growth mechanism of Ni0.3Mn0.7CO3 precursor for high capacity Li-ion battery cathodes. J. Mater. Chem. 21, 9290–9295. doi:10.1039/c1jm11077b
Wang, J., Qiu, B., Cao, H., Xia, Y., and Liu, Z. (2012). Electrochemical properties of 0.6 Li[Li1/3Mn2/3]O2-0.4LiNixMnyCo1-x-yO2 cathode materials for lithium-ion batteries. J. Power Sources. 218, 128–133. doi:10.1016/j.jpowsour.2012.06.067
Wang, Z., Liu, E., He, C., Shi, C., Li, J., and Zhao, N. (2013). Effect of amorphous FePO4 coating on structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J. Power Sources 236, 25–32. doi:10.1016/j.jpowsour.2013.02.022
Wei, X., Zhang, S., He, L., Liu, G., and Yang, P. (2013). Structure, morphology and electrochemical properties of Li[Li0.2Co0.4Mn0.4]O2 cathode material synthesized by a simple hydrothermal method. Int. J. Electrochem. Sci. 8, 1885–1894.
Wu, F., Li, N., Su, Y., Lu, H., Zhang, L., An, R., et al. (2012). Can surface modification be more effective to enhance the electrochemical performance of lithium rich materials? J. Mater. Chem. 22, 1489–1497. doi:10.1039/c1jm14459f
Wu, F., Li, N., Su, Y., Zhang, L., Bao, L., Wang, J., et al. (2014). Ultrathin spinel membrane-encapsulated layered lithium-rich cathode material for advanced Li-ion batteries. Nano Lett. 14, 3550–3555. doi:10.1021/nl501164y
Wu, F., Lu, H., Su, Y., Li, N., Bao, L., and Chen, S. (2010). Preparation and electrochemical performance of Li-rich layered cathode material, Li[Ni0.2Li0.2Mn0.6]O2, for lithium-ion batteries. J. Appl. Electrochem. 40, 783–789. doi:10.1007/s10800-009-0057-2
Wu, X., Ryu, K. S., Hong, Y.-S., Park, Y. J., and Chang, S. H. (2004). Properties of Li[CrxLi(1-x)/3Mn2(1-x)/3]O2 (0.1 ≤ x ≤ 0.2) material prepared by quenching. J. Power Sources 132, 219–224. doi:10.1016/j.jpowsour.2003.12.030
Wu, Y., and Manthiram, A. (2009). Effect of surface modifications on the layered solid solution cathodes (1-z)Li[Li1/3Mn2/3]O2-(z) Li[Mn0.5 - yNi0.5 - yCo2y]O2. Solid State Ionics 180, 50–56. doi:10.1016/j.ssi.2008.11.002
Xiang, X., Li, X., and Li, W. (2013). Preparation and characterization of size-uniform Li[Li0.131Ni0.304Mn0.565]O2 particles as cathode materials for high energy lithium ion battery. J. Power Sources 230, 89–95. doi:10.1016/j.jpowsour.2012.12.050
Xu, B., Fell, C. R., Chic, M., and Meng, Y. S. (2011). Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: a joint experimental and theoretical study. Energy Environ. Sci. 4, 2223–2233. doi:10.1039/c1ee01131f
Ying, J., Jiang, C., and Wan, C. (2004). Preparation and characterization of high-density spherical LiCoO2 cathode material for lithium ion batteries. J. Power Sources 129, 264–269. doi:10.1016/j.jpowsour.2003.10.007
Yu, H., Ishikawa, R., So, Y.-G., Shibata, N., Kudo, T., Zhou, H., et al. (2013a). Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries. Angew. Chem. 125, 6085–6089. doi:10.1002/anie.201301236
Yu, H., Wang, Y., Asakura, D., Hosono, E., Zhang, T., and Zhou, H. (2012a). Electrochemical kinetics of the 0.5Li2MnO3.0.5LiMn0.42Ni0.42Co0.16O2 ‘composite’ layered cathode material for lithium-ion batteries. RSC Adv. 2, 8797–8807. doi:10.1039/c2ra20772a
Yu, H., Kim, H., Wang, Y., He, P., Asakura, D., Nakamura, Y., et al. (2012b). High-energy ‘composite’ layered manganese-rich cathode materials via controlling Li2MnO3 phase activation for lithium-ion batteries. Phys. Chem. Chem. Phys. 14, 6584–6595. doi:10.1039/c2cp40745k
Yu, H., and Zhou, H. (2012). Initial coulombic efficiency improvement of the Li1.2Mn0.567Ni0.166Co0.067O2 lithium-rich material by ruthenium substitution for manganese. J. Mater. Chem. 22, 15507–15510. doi:10.1039/c2jm33484d
Yu, H., and Zhou, H. (2013). High-energy cathode materials (Li2MnO3-LiMO2) for lithium-ion batteries. J. Phys. Chem. Lett. 4, 1268–1280. doi:10.1002/chem.201402727
Yu, S.-H., Yoon, T., Mun, J., Park, S., Kang, Y.-S., Park, J.-H., et al. (2013b). Continuous activation of Li2MnO3 component upon cycling in Li1.167Ni0.233Co0.100Mn0.467Mo0.033O2 cathode material for lithium ion batteries. J. Mater. Chem. A 1, 2833–2839. doi:10.1039/c2ta00309k
Zhang, H. Z., Qiao, Q. Q., Li, G. R., Ye, S. H., and Gao, X. P. (2012). Surface nitridation of Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as cathode material for lithium-ion battery. J. Mater. Chem. 22, 13104–13109. doi:10.1039/c2jm30989k
Zhang, X., and Axelbaum, R. L. (2012). Spray pyrolysis synthesis of mesoporous lithium-nickel-manganese-oxides for high energy Li-ion batteries. J. Electrochem. Soc. 159, A834–A842. doi:10.1149/2.079206jes
Zhao, Y., Zhao, C., Feng, H., Sun, Z., and Xia, D. (2011). Enhanced electrochemical performance of Li[Li0.2Ni0.2Mn0.6]O2 modified by manganese oxide coating for lithium-ion batteries. Electrochem. Solid State Lett. 14, A1–A5. doi:10.1149/1.3496402
Keywords: cathode materials, lithium-rich layered oxides, powders, lithium-ion batteries, synthesis
Citation: Doan TNL, Yoo K, Hoang TKA and Chen P (2014) Recent developments in synthesis of xLi2MnO3 · (1 − x)LiMO2 (M = Ni, Co, Mn) cathode powders for high-energy lithium rechargeable batteries. Front. Energy Res. 2:36. doi: 10.3389/fenrg.2014.00036
Received: 12 June 2014; Paper pending published: 03 July 2014;
Accepted: 16 August 2014; Published online: 01 September 2014.
Edited by:
Xueliang Sun, Western University, CanadaReviewed by:
Reza Younesi, Technical University of Denmark, DenmarkZhaoxiang Wang, Chinese Academy of Sciences, China
Hao Liu, Chinese Academy of Engineering Physics, China
Copyright: © 2014 Doan, Yoo, Hoang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: P. Chen, Department of Chemical Engineering and Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue West, Waterloo, ON N2L3G1, Canada e-mail: p4chen@uwaterloo.ca
 The Nam Long Doan
The Nam Long Doan Kimoon Yoo
Kimoon Yoo
 Tuan K. A. Hoang
Tuan K. A. Hoang P. Chen
P. Chen