Forever young: rejuvenating muscle satellite cells
- 1Epigenetics and Regenerative Medicine, IRCCS Fondazione Santa Lucia, Rome, Italy
- 2Institute of Cell Biology and Neurobiology, National Research Council of Italy, Rome, Italy
- 3Institute of Translational Pharmacology, National Research Council of Italy, Rome, Italy
A hallmark of aging is alteration of organismal homeostasis and progressive decline of tissue functions. Alterations of both cell intrinsic functions and regenerative environmental cues contribute to the compromised stem cell activity and reduced regenerative capability occurring in aged muscles. In this perspective, we discuss the new evidence supporting the hypothesis that skeletal muscle stem cells (MuSCs) are intrinsically defective in elderly muscles. In particular, we review three recent papers leading to identify fibroblast growth factor receptor-1, p38 mitogen-activated protein kinase, and p16INK4a as altered signaling in satellite cells from aged mice. These pathways contribute to age-related loss of MuSCs asymmetric polarization, compromised self-renewal capacity, and acquisition of pre-senescent state. The pharmacological manipulation of those networks can open novel strategies to rejuvenate MuSCs and counteract the functional decline of skeletal muscle during aging.
Extended lifespan raises the issue of handling age-related disorders, which profoundly affect the quality of life of an increasing number of people. At the physiological level, the most relevant feature of aging is the functional decline of tissue functions (Oh et al., 2014).
In particular, in the elderly, muscle mass declines progressively by means of a process named sarcopenia, making skeletal muscle one of the more compromised tissues during aging. Beyond the protein breakdown associated with the loss of sarcomeric proteins, aged muscles display compromised regenerative capacity associated with altered environmental cues (Kim and Choi, 2013; Sayer et al., 2013).
Muscle regeneration is achieved by the interplay between adult stem cells, named muscle satellite cells (MuSCs), and other cellular types (i.e., macrophages and muscle interstitial cells) that participate in the orchestration of regeneration. Muscle niche derived and systemic cues contribute to regulate muscle homeostasis and functionality (Chakkalakal et al., 2012; Bentzinger et al., 2013). Changes of those three compartments are described throughout lifetime and account for the decline of functional capacities in the elderly (Jang et al., 2011). Upon muscle injury, MuSCs that are located in a niche between the basal lamina and the sarcolemma, become activated and recapitulate myogenic differentiation to replenish damaged muscle (Collins et al., 2005; Cheung and Rando, 2013). Additionally, environmental cues finely regulate this process driving efficient muscle regeneration (Sinha et al., 2014). In order to ensure optimal performance, it is critical that several properties of MuSCs are finely regulated and coordinated. Amongst these properties are survival, self-renewal, fine-tuning between exit from quiescence and proliferative expansion, and eventually commitment toward myogenic differentiation (Bentzinger et al., 2013). All these processes are altered in the elderly leading to compromised muscle functionality.
Beyond the notion provided by parabiosis experiments that circulating systemic factors are able to restore muscle regeneration in aged mice (Conboy et al., 2005), recent evidence supports the hypothesis that MuSCs are intrinsically defective in aged muscles. These new findings open the possibility to target this stem cell compartment to counteract functional decline of muscle during aging. Here, we will provide a general comment on three breakthrough studies from Bernet et al. (2014), Sousa-Victor et al. (2014) and Cosgrove et al. (2014) discussing the relative contribution to muscle regeneration of cell-autonomous vs. cell non-autonomous factors during aging.
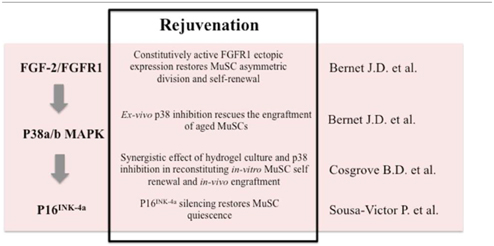
In their work Bernet et al. and Cosgrove et al. provide evidence that constitutive activation of the p38 MAPK in aged MuSCs leads to a decline in their self-renewal and regenerative capacity. Both groups demonstrated that partial pharmacological inhibition of p38 is sufficient to restore the ability of MuSCs to participate efficiently in muscle regeneration and to maintain the stem cell pool. Interestingly, Bernet et al. identify an alteration of the FGF-2/FGFR1 axis as a feature of aged MuSC dysfunction, as observed previously by Brack and colleagues (Chakkalakal et al., 2012). Although in the paper by Chakkalakal the authors suggest that increased activity of FGFR1 results in the disruption of MuSC quiescence in aged muscles, the Bernet study supports the hypothesis that FGF-2 increase in the aged niche is a compensatory response to the loss of function of FGFR1 activity observed in aged MuSCs. In particular, they show that while in young MuSCs the FGF2/FGFR1 axis drives asymmetric division through activation of p38 only in the committed daughter cell, in aged MuSCs this balance is altered. Indeed, the insensitivity to FGF signaling in the elderly MuSCs results in constitutive activation of p38 with loss of asymmetric polarization and impaired self-renewal capacity. Likewise, FGFR1 ligand independent, constitutive activation restores MuSC asymmetric cell division.
With elegant experiments of autologous and serial MuSC transplantation Cosgrove et al. demonstrate the intrinsic defect of elderly derived MuSCs in association with increased p38 activity. The authors demonstrate a synergistic interaction of biochemical and biophysical factors, respectively pharmacological inhibition of p38 and a hydrogel culture system, which contribute to reconstitute the proliferative capability and self-renewal as assayed by in vitro and in vivo engraftment. The effect of p38 inhibition in driving stem cell renewal was already demonstrated by Palacios et al. (2010), supporting the notion that pharmacological intervention with p38 inhibitors may support muscle regeneration. Moreover, this paper provides a useful strategy to overcome the bottleneck of in vitro stem cell expansion in cell therapies using specific soft biomaterial that mimics the muscle niche.
In the same month Sousa-Victor and colleagues came out with a study demonstrating that geriatric MuSCs fail to support muscle regeneration and display defective activation. Serial transplantation experiments supported the conclusion that this defect is a cell intrinsic feature of geriatric MuSCs. They identify the master regulator of senescence p16INK4a as a key determinant responsible for a quiescence-senescence switch (a process named geroconversion) operating in geriatric MuSCs in coincidence with their impaired regenerative potential. Indeed, genetic inactivation of p16INK4a locus was sufficient to recover the cells from the senescence-associated cell cycle arrest and restore their self-renewal capacity, leading to the reconstitution of the stem cell pool after muscle damage. The novelty of this study relies on the finding that geriatric stem cells are associated with the progressive accumulation of DNA damage and senescence-associated markers that in turn contribute to the loss of reversible quiescence mediated by p16INK4a. Indeed, in geriatric MuSCs, the p16INK4a locus is constitutively de-repressed due to altered PRC1 complex function.
These studies demonstrate that in addition to the regenerative environment that profoundly affects the niche and stem cell function, there is another level of tissue homeostasis regulation that is intrinsic to adult stem cells. The cell autonomous functionality declines in the elderly due to de-regulated p38 signaling and accumulation of DNA damage and senescence-associated features. This evidence suggests new avenues to reverse the dysfunctional status of MuSCs from aged tissues. For instance, constitutive FGFR1 signaling can restore MuSCs asymmetric division and self-renewal, and pharmacological blockade of p38 signaling can promote MuSCs self-renewal and engraftment by silencing p16INK4a, thus reversing geroconversion and allowing MuSCs to support muscle regeneration (Table 1). Intriguingly, the activation of p38 signaling has been associated with senescence (Wang et al., 2002) as well as increasing levels of p16INK4a (Serrano et al., 1997; Iwasa et al., 2003) in cell types other than muscle stem cells highlighting the notion that a more complex signaling network that may be context dependent controls senescence (Xu et al., 2014). The p38 signaling pathway has been demonstrated to be involved in IL-6 induced STAT3 transcriptional activation (Zauberman et al., 1999; Riebe et al., 2011). Intriguingly, the recent finding that increases in JAK-STAT signaling inhibits MuSCs function during aging further provides evidence for the pivotal role of p38 in driving muscle regeneration (Price et al., 2014; Tierney et al., 2014). Future studies should determine the molecular relationship between these new players of muscle aging—DNA damage, p38 signaling and p16INK4a in order to devise treatments aimed at reversing MuSC senescence.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bentzinger, C. F., Wang, Y. X., Dumont, N. A., and Rudnicki, M. A. (2013). Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 14, 1062–1072. doi: 10.1038/embor.2013.182
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bernet, J. D., Doles, J. D., Hall, J. K., Kelly Tanaka, K., Carter, T. A., and Olwin, B. B. (2014). p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 20, 265–271. doi: 10.1038/nm.3465
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chakkalakal, J. V., Jones, K. M., Basson, M. A., and Brack, A. S. (2012). The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360. doi: 10.1038/nature11438
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cheung, T. H., and Rando, T. A. (2013). Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14, 329–340. doi: 10.1038/nrm3591
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Collins, C. A., Olsen, I., Zammit, P. S., Heslop, L., Petrie, A., Partridge, T. A., et al. (2005). Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301. doi: 10.1016/j.cell.2005.05.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Conboy, I. M., Conboy, M. J., Wagers, A. J., Girma, E. R., Weissman, I. L., and Rando, T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. doi: 10.1038/nature03260
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cosgrove, B. D., Gilbert, P. M., Porpiglia, E., Mourkioti, F., Lee, S. P., Corbel, S. Y., et al. (2014). Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264. doi: 10.1038/nm.3464
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Iwasa, H., Han, J., and Ishikawa, F. (2003). Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells 8, 131–144. doi: 10.1046/j.1365-2443.2003.00620.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jang, Y. C., Sinha, M., Cerletti, M., Dall'Osso, C., and Wagers, A. J. (2011). Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb. Symp. Quant. Biol. 76, 101–111. doi: 10.1101/sqb.2011.76.010652
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, T. N., and Choi, K. M. (2013). Sarcopenia: definition, epidemiology, and pathophysiology. J. Bone Metab. 20, 1–10. doi: 10.11005/jbm.2013.20.1.1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oh, J., Lee, Y. D., and Wagers, A. J. (2014). Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20, 870–880. doi: 10.1038/nm.3651
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Palacios, D., Mozzetta, C., Consalvi, S., Caretti, G., Saccone, V., Proserpio, V., et al. (2010). TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 7, 455–469. doi: 10.1016/j.stem.2010.08.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Price, F. D., von Maltzahn, J., Bentzinger, C. F., Dumont, N. A., Yin, H., Chang, N. C., et al. (2014). Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 20, 1174–1181. doi: 10.1038/nm.3655
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Riebe, C., Pries, R., Schroeder, K. N., and Wollenberg, B. (2011). Phosphorylation of STAT3 in head and neck cancer requires p38 MAPKinase, whereas phosphorylation of STAT1 occurs via a different signaling pathway. Anticancer Res. 31, 3819–3825.
Sayer, A. A., Robinson, S. M., Patel, H. P., Shavlakadze, T., Cooper, C., and Grounds, M. D. (2013). New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing 42, 145–150. doi: 10.1093/ageing/afs191
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D., and Lowe, S. W. (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602. doi: 10.1016/S0092-8674(00)81902-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sinha, M., Jang, Y. C., Oh, J., Khong, D., Wu, E. Y., Manohar, R., et al. (2014). Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649–652. doi: 10.1126/science.1251152
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sousa-Victor, P., Gutarra, S., Garcia-Prat, L., Rodriguez-Ubreva, J., Ortet, L., Ruiz-Bonilla, V., et al. (2014). Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321. doi: 10.1038/nature13013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tierney, M. T., Aydogdu, T., Sala, D., Malecova, B., Gatto, S., Puri, P. L., et al. (2014). STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 20, 1182–1186. doi: 10.1038/nm.3656
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, W., Chen, J. X., Liao, R., Deng, Q., Zhou, J. J., Huang, S., et al. (2002). Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell. Biol. 22, 3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xu, Y., Li, N., Xiang, R., and Sun, P. (2014). Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci. 39, 268–276. doi: 10.1016/j.tibs.2014.04.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zauberman, A., Zipori, D., Krupsky, M., and Ben-Levy, R. (1999). Stress activated protein kinase p38 is involved in IL-6 induced transcriptional activation of STAT3. Oncogene 18, 3886–3893. doi: 10.1038/sj.onc.1202738
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: muscle satellite cells, muscle regeneration, muscle aging, p38 mitogen-activated protein kinases, p16INK4a
Citation: Madaro L and Latella L (2015) Forever young: rejuvenating muscle satellite cells. Front. Aging Neurosci. 7:37. doi: 10.3389/fnagi.2015.00037
Received: 16 January 2015; Accepted: 04 March 2015;
Published: 21 April 2015.
Edited by:
Pura Muñoz-Cánoves, Pompeu Fabra University, SpainReviewed by:
Penney M. Gilbert, University of Toronto, CanadaEusebio Perdiguero, University Pompeu Fabra, Spain
Copyright © 2015 Madaro and Latella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Latella, Institute of Translational Pharmacology, National Research Council of Italy, Via del Fosso dl Cavaliere 100, 00133 Rome, Italy l.latella@hsantalucia.it; lucia.latella@ift.cnr.it
 Luca Madaro
Luca Madaro Lucia Latella
Lucia Latella