- Department of Anatomy and Neurosciences, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, Netherlands
Functional Reorganization in MS: An Outdated Concept?
The current field of multiple sclerosis (MS) research is an active and highly interesting one: structural abnormalities such as inflammatory lesions and brain atrophy are studied with a wide array of advanced neuroimaging techniques (1). These techniques are subsequently used to try to explain the large clinical heterogeneity in patients. Clinically important in MS is cognitive dysfunction, which is present in 40–70% of all patients (2, 3). Cognitive impairment in MS receives much attention, as there is currently no proven effective treatment, but symptoms may nevertheless start in early stages of disease already (4). Cognitive decline is known to exert deleterious effects on psychosocial functioning (2, 5, 6). Traditional structural imaging measures like lesion volumes are notoriously poorly related with cognitive function (7), so a move toward more sensitive, comprehensive measures is required, such as those that measure brain function in addition to brain structure.
Historically, most early imaging studies have used the paced auditory serial addition test (PASAT) to study cognition in MS, a task that measures information processing speed (8–10). These observed a combination of hyperactivation of frontal regions in response to the task and a recruitment of additional areas, not normally attributed to the task in controls. The functional changes were mostly positively related to the amount of structural damage in the brain, and were stronger in patients who scored normally on the PASAT, indicating that it might be a beneficial process. Later studies investigated other cognitive domains and also showed such an apparently beneficial increased local activation, for example, during a memory task in the hippocampus (11) and during the N-back working memory task in the dorsolateral prefrontal cortex (DLPFC) (12). Importantly, these studies also showed decreased activation in cognitively impaired patients.
The body of literature of that point in time led to our previous hypothesis of functional reorganization in MS (13). This hypothesis asserted that a “compensatory” change is seen in the brains of MS patients in the form of an increase in brain function, i.e., both increased activation and increased connectivity. Functional connectivity is conceptually quite different from task-based activation and reflects the amount of communication between brain regions, i.e., coherent patterns of firing typically measured with correlation measures. Early connectivity studies investigated the so-called “default mode network” (DMN), which is only coherently active during a resting state. Two such studies found DMN changes that were interpreted in the same way as the task-based activation studies: increased DMN connectivity in clinically isolated syndrome (CIS) patients (14) and decreased DMN connectivity in progressive MS, which was related to cognitive impairment (15). We proposed that increasing structural damage, in combination with an optimum curve of “functional reorganization,” results in a delayed, non-linear, development of cognitive dysfunction.
However, the previous model was mostly based on task-based activation studies, while the connectivity field was still in its infancy. As the concept of functional reorganization was gaining support, the field was primed for finding cognitively relevant connectivity changes. Interestingly, recent studies have mostly related increased functional connectivity to cognitive dysfunction, raising doubts on the previous concept of functional reorganization in MS. In this paper, we will review this recent functional connectivity literature and reiterate the case around functional connectivity changes in MS and their potential effects on cognition. Which reported connectivity changes can be justifiably said to be “compensatory” or “beneficial”? Which are likely “maladaptive”? Can any such predicate be arrived at all, based on the neuroscientific studies available? Is it perhaps time to revise our previous model of functional reorganization?
Functional Connectivity in MS: A Field of Contradictions
Resting state network changes have been observed in relapsing remitting MS (RRMS) patients, both within and between almost all resting state sub-networks (16). The DMN de-activates when performing a task, and appears to be strongly related to cognition. DMN changes have been difficult to place within our previous hypothesis, as cognitive dysfunction was related to both decreased (17–21) and increased DMN connectivity (22–24). In pediatric MS, increased DMN connectivity was seen in cognitively preserved patients in the anterior cingulate gyrus, while decreased connectivity of the posterior cingulate was seen in cognitively impaired patients (25). Increased connectivity of the anterior cingulate cortex was also found in adult MS patients, although these connectivity changes showed both positive and negative correlations with cognitive dysfunction (26). Another recent paper in adult-onset MS suggests that the severity of cognitive impairment is directly related to the level of increased functional connectivity of the DMN (27). As the DMN de-activates during tasks, task-based studies have also looked at this network. During performance of the N-back working memory task, researchers noted less de-activation of the DMN (12) in cognitively impaired patients. Another recent study, however, seems to contradict this finding, as an increased DMN activation during a similar task was related to both higher intellectual enrichment and information processing speed performance (28). In short, the DMN results have been difficult to interpret.
Unfortunately, results from seed-based analyses investigating other structures like the DLPFC have not been very consistent either. One such study (29) found a reduced connectivity between the DLPFC and the superior medial frontal gyrus in patients who scored normally on the N-back, in relation to increased difficulty of the task, and also found increased connectivity between the left and right prefrontal cortices. This connectivity between the DLPFC and medial frontal regions was increased in MS patients in another study, during the Go/No Go task, at which they were impaired (30). The DLPFC was also studied during performance of the PASAT in patients with CIS who were impaired on this test (31, 32), showing decreased connectivity with several areas, including the anterior cingulate and thalamus. Contrarily, another study only showed increased connectivity during the PASAT in CIS patients, who were also impaired on this test (33).
Studies looking at several other cognitively relevant structures such as the thalamus, hippocampus, and cerebellum have shown varying patterns of connectivity in MS as well. Thalamic atrophy has well-known and strong effects on cognition in MS (34), which appears related to global cortical network changes (24, 35). An aforementioned task-based CIS study showed decreased connectivity between the thalamus and DLPFC during the PASAT (31), at which patients were impaired. Strikingly, during a resting state, the thalamus has also been shown to have increased connectivity with frontal areas in clinically definite MS patients with cognitive impairment (36, 37). Similarly, at rest, the hippocampus showed decreased connectivity related to hippocampal atrophy in patients with still intact memory performance (38), but increased connectivity in patients with memory impairment (39). The cerebellum, however, showed decreased connectivity in patients with cognitive dysfunction, both during the PASAT (40) and Stroop tasks (41).
What Does it all Mean?
As described above, the body of literature on cognitively relevant connectivity changes in MS is currently difficult to interpret. As it seems, our previous model for functional reorganization is incomplete and the term is currently used in a number of ways and lacks a clear definition. Additionally, these findings were studied across the spectrum of clinical and cognitive phenotypes in MS, with very different methodological and statistical approaches, leaving the data ambiguous in places. Some studies now refer to any connectivity change as functional reorganization, leaving it to the reader to disentangle “beneficial” or “maladaptive” functional reorganization post hoc. This process actually seems quite complicated, however, as cross-sectional studies have related both connectivity increases and decreases to cognitive dysfunction in MS. Therefore, the studies that do claim that changes might be beneficial for cognitive performance in MS might not have enough evidence to do so. In truth, we are currently unable to disentangle “good” from “bad” and are strongly limited by the cross-sectional nature of almost all of these studies.
For example, suppose that a functional connectivity increase is observed in cognitively preserved patients, and a decrease in a cognitively impaired patient group. Although many studies interpret such a finding as cognitively relevant, as described previously, such data could, in fact, be interpreted in several ways. First, the functional connectivity increase in cognitively preserved patients might reflect “beneficial” functional reorganization, delaying cognitive impairment. In impaired patients, this effect of functional reorganization is then lost. Second, the functional connectivity increase in cognitively preserved patients might be a “maladaptive” response, following, e.g., disinhibition, heralding an imminent network collapse, and further deterioration into cognitive impairment. Third, the functional connectivity increase in cognitively preserved patients could be an unrelated epiphenomenon. Or, that the connectivity increase is related to structural damage, but that it has no direct impact on cognition at all. And finally, given the fact that most studies are cross-sectional, it cannot be excluded that the frequently observed functional connectivity increases in patients with cognitive impairment are, in fact, “beneficial.” It is possible that such increases are, e.g., a bleed through of beneficial functional reorganization from the cognitively preserved stage. This could be due to a poor definition of cognitive impairment and/or plastic changes that persist throughout this stage of the disease. The only way we are going to understand the cognitive role of functional connectivity changes in MS will be to study them over time.
Preliminary longitudinal studies linking connectivity changes to cognitive rehabilitation (42, 43), as well as pharmacological intervention (44), show some promise. Unfortunately, determining sufficient sample sizes and time frames remains difficult given the current lack of data, leaving these small studies difficult to interpret. Such intervention studies aiming to increase neurotransmitter levels in MS appear logical, as there is an apparent cholinergic (45) as well as glutamate (46) imbalance in MS, which might leave the network unstable. Therefore, pharmacological therapies targeting such neurotransmitters might prove valuable (47). It must be stressed, however, that there may also be downsides to such an approach, as specific glutamate receptor subtypes have been linked to brain atrophy (48) and excitotoxic effects due to the treatment and the functional reorganization process might actually increase tissue damage and network stress.
The Future: Measuring Network Collapse in MS
As the field of functional imaging in MS matured, the clinical interpretation of the combined set of functional changes in MS has become much more complex, leaving our previous model of functional reorganization in MS incomplete and too simplistic. After exploring abovementioned individual structures and sub-networks in MS has not made matters much clearer, it is now opportune to look at connectivity in another way. One option is to take functional connectivity values and convert them into a more holistic network model of the entire brain. This so-called graph analysis approach (49) uses different parameters such as the clustering coefficient and path length (50) to describe network information flow. Applications of these techniques in MS have been very limited (49), but have highlighted the power of graph analysis in discriminating patients from controls (51). Graph analytical studies in MS have shown that cognitive dysfunction is related to an inefficient network, as seen by the change in clustering coefficient and path length (52–54), impaired network integration of information (55) and clustering (56), decreases in network centrality (57, 58), increases in modularity (59), and changes in minimum spanning tree parameters (35, 60). These graph measures provide us many new ways to conceptualize and understand what actually happens to the global status of the entire brain network in patients with cognitive impairment in MS, beyond the poorly understood local increases or decreases in connectivity. Future longitudinal studies are now required to assess the predictive power of these measures. Together, it appears that the brain network of patients with cognitive impairment in MS features a strong decrease in whole-network efficiency, i.e., a network “collapse” (see Figure 1).
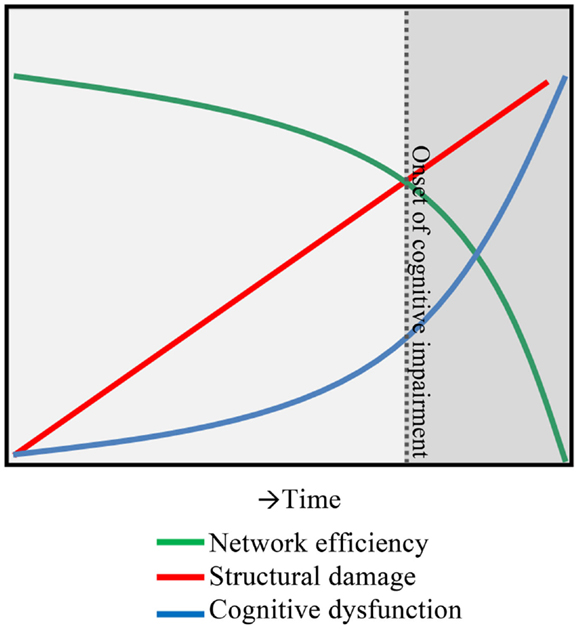
Figure 1. A hypothesis of network collapse as a cause for developing cognitive impairment in MS. In early stages of MS, structural damage is low, leaving network efficiency relatively high. As the structural damage accumulates over time, network efficiency levels drop, inducing a network collapse after a critical threshold (indicated by the dotted line) is exceeded. After this, the network is unable to function normally and cognitive impairment develops.
In summary, thinking about functional reorganization processes and labeling them as either “beneficial” or “maladaptive” has proven to be overly simplistic. A more holistic approach is required, encompassing both activation and connectivity data into a frame of network dynamics in a longitudinal fashion. Following this, first steps toward using more sophisticated (functional) imaging tools to monitor cognitive deficits can hopefully be taken.
Author Contributions
All authors contributed to the conception, drafting, revising, and finalizing of the manuscript and agree to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The MS Center Amsterdam is supported by the Dutch MS Research Foundation, grant numbers 13-820 and 14-358e.
References
1. Filippi M, Grossman RI. MRI techniques to monitor MS evolution – the present and the future. Neurology (2002) 58(8):1147–53. doi: 10.1212/WNL.58.8.1147
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology (1991) 41(5):685–91. doi:10.1212/WNL.41.5.685
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol (2008) 7(12):1139–51. doi:10.1016/S1474-4422(08)70259-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Amato MP, Portaccio E, Goretti B, Zipoli V, Hakiki B, Giannini M, et al. Cognitive impairment in early stages of multiple sclerosis. Neurol Sci (2010) 31(Suppl 2):S211–4. doi:10.1007/s10072-010-0376-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Mitchell AJ, Kemp S, Benito-Leon J, Reuber M. The influence of cognitive impairment on health-related quality of life in neurological disease. Acta Neuropsychiatr (2010) 22(1):2–13. doi:10.1111/j.1601-5215.2009.00439.x
6. Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology (1991) 41(5):692–6. doi:10.1212/WNL.41.5.692
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol (2004) 61(2):226–30. doi:10.1001/archneur.61.2.226
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Staffen W, Mair A, Zauner H, Unterrainer J, Niederhofer H, Kutzelnigg A, et al. Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain (2002) 125(Pt 6):1275–82. doi:10.1093/brain/awf125
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Audoin B, Ibarrola D, Ranjeva JP, Confort-Gouny S, Malikova I, Ali-Chérif A, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp (2003) 20(2):51–8. doi:10.1002/hbm.10128
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Mainero C, Caramia F, Pozzilli C, Pisani A, Pestalozza I, Borriello G, et al. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage (2004) 21(3):858–67. doi:10.1016/j.neuroimage.2003.10.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Hulst HE, Schoonheim MM, Roosendaal SD, Popescu V, Schweren LJ, van der Werf YD, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp (2012) 33(10):2268–80. doi:10.1002/hbm.21359
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Rocca MA, Valsasina P, Hulst HE, Abdel-Aziz K, Enzinger C, Gallo A, et al. Functional correlates of cognitive dysfunction in multiple sclerosis: a multicenter fMRI Study. Hum Brain Mapp (2014) 35(12):5799–814. doi:10.1002/hbm.22586
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Schoonheim MM, Geurts JJ, Barkhof F. The limits of functional reorganization in multiple sclerosis. Neurology (2010) 74(16):1246–7. doi:10.1212/WNL.0b013e3181db9957
14. Roosendaal SD, Schoonheim MM, Hulst HE, Sanz-Arigita EJ, Smith SM, Geurts JJ, et al. Resting state networks change in clinically isolated syndrome. Brain (2010) 133(Pt 6):1612–21. doi:10.1093/brain/awq058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology (2010) 74(16):1252–9. doi:10.1212/WNL.0b013e3181d9ed91
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Rocca MA, Valsasina P, Martinelli V, Misci P, Falini A, Comi G, et al. Large-scale neuronal network dysfunction in relapsing-remitting multiple sclerosis. Neurology (2012) 79(14):1449–57. doi:10.1212/WNL.0b013e31826d5f10
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Cruz-Gomez AJ, Ventura-Campos N, Belenguer A, Avila C, Forn C. The link between resting-state functional connectivity and cognition in MS patients. Mult Scler (2014) 20(3):338–48. doi:10.1177/1352458513495584
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Louapre C, Perlbarg V, García-Lorenzo D, Urbanski M, Benali H, Assouad R, et al. Brain networks disconnection in early multiple sclerosis cognitive deficits: an anatomofunctional study. Hum Brain Mapp (2014) 35(9):4706–17. doi:10.1002/hbm.22505
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Bonavita S, Gallo A, Sacco R, Corte MD, Bisecco A, Docimo R, et al. Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler (2011) 17(4):411–22. doi:10.1177/1352458510394609
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Leavitt VM, Paxton J, Sumowski JF. Default network connectivity is linked to memory status in multiple sclerosis. J Int Neuropsychol Soc (2014) 20(9):937–44. doi:10.1017/S1355617714000800
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Wojtowicz M, Mazerolle EL, Bhan V, Fisk JD. Altered functional connectivity and performance variability in relapsing-remitting multiple sclerosis. Mult Scler (2014) 20(11):1453–63. doi:10.1177/1352458514524997
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Faivre A, Rico A, Zaaraoui W, Crespy L, Reuter F, Wybrecht D, et al. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult Scler (2012) 18(9):1251–8. doi:10.1177/1352458511435930
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Zhou F, Zhuang Y, Gong H, Wang B, Wang X, Chen Q, et al. Altered inter-subregion connectivity of the default mode network in relapsing remitting multiple sclerosis: a functional and structural connectivity study. PLoS One (2014) 9(7):e101198. doi:10.1371/journal.pone.0101198
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Tewarie P, Schoonheim MM, Stam CJ, van der Meer ML, van Dijk BW, Barkhof F, et al. Cognitive and clinical dysfunction, altered MEG resting-state networks and thalamic atrophy in multiple sclerosis. PLoS One (2013) 8(7):e69318. doi:10.1371/journal.pone.0069318
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Rocca MA, Absinta M, Amato MP, Moiola L, Ghezzi A, Veggiotti P, et al. Posterior brain damage and cognitive impairment in pediatric multiple sclerosis. Neurology (2014) 82(15):1314–21. doi:10.1212/WNL.0000000000000309
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Loitfelder M, Filippi M, Rocca M, Valsasina P, Ropele S, Jehna M, et al. Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PLoS One (2012) 7(8):e42862. doi:10.1371/journal.pone.0042862
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A (2011) 108(47):19066–71. doi:10.1073/pnas.1110024108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain (2010) 133(Pt 2):362–74. doi:10.1093/brain/awp307
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain (2006) 129(Pt 2):527–37. doi:10.1093/brain/awh670
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Bonnet MC, Allard M, Dilharreguy B, Deloire M, Petry KG, Brochet B. Cognitive compensation failure in multiple sclerosis. Neurology (2010) 75(14):1241–8. doi:10.1212/WNL.0b013e3181f612e3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Ranjeva JP, Audoin B, Au Duong MV, Confort-Gouny S, Malikova I, Viout P, et al. Structural and functional surrogates of cognitive impairment at the very early stage of multiple sclerosis. J Neurol Sci (2006) 245(1–2):161–7. doi:10.1016/j.jns.2005.09.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Au Duong MV, Audoin B, Boulanouar K, Ibarrola D, Malikova I, Confort-Gouny S, et al. Altered functional connectivity related to white matter changes inside the working memory network at the very early stage of MS. J Cereb Blood Flow Metab (2005) 25(10):1245–53. doi:10.1038/sj.jcbfm.9600122
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Forn C, Rocca MA, Valsasina P, Boscá I, Casanova B, Sanjuan A, et al. Functional magnetic resonance imaging correlates of cognitive performance in patients with a clinically isolated syndrome suggestive of multiple sclerosis at presentation: an activation and connectivity study. Mult Scler (2012) 18(2):153–63. doi:10.1177/1352458511417744
34. Schoonheim MM, Popescu V, Rueda Lopes FC, Wiebenga OT, Vrenken H, Douw L, et al. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology (2012) 79(17):1754–61. doi:10.1212/WNL.0b013e3182703f46
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Tewarie P, Schoonheim MM, Schouten DI, Polman CH, Balk LJ, Uitdehaag BM, et al. Functional brain networks: linking thalamic atrophy to clinical disability in multiple sclerosis, a multimodal fMRI and MEG study. Hum Brain Mapp (2014) 36(2):603–18. doi:10.1002/hbm.22650
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Tona F, Petsas N, Sbardella E, Prosperini L, Carmellini M, Pozzilli C, et al. Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology (2014) 271(3):814–21. doi:10.1148/radiol.14131688
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Schoonheim MM, Hulst HE, Brandt RB, Strik M, Wink AM, Uitdehaag BM, et al. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology (2015) 84(8):776–83. doi:10.1212/WNL.0000000000001285
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Roosendaal SD, Hulst HE, Vrenken H, Feenstra HE, Castelijns JA, Pouwels PJ, et al. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology (2010) 255(2):595–604. doi:10.1148/radiol.10091433
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Hulst HE, Schoonheim MM, Van Geest Q, Uitdehaag BM, Barkhof F, Geurts JJ. Memory impairment in multiple sclerosis: relevance of hippocampal activation and hippocampal connectivity. Mult Scler (2015). doi:10.1177/1352458514567727
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Cerasa A, Passamonti L, Valentino P, Nisticò R, Pirritano D, Gioia MC, et al. Cerebellar-parietal dysfunctions in multiple sclerosis patients with cerebellar signs. Exp Neurol (2012) 237(2):418–26. doi:10.1016/j.expneurol.2012.07.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Rocca MA, Bonnet MC, Meani A, Valsasina P, Colombo B, Comi G, et al. Differential cerebellar functional interactions during an interference task across multiple sclerosis phenotypes. Radiology (2012) 265(3):864–73. doi:10.1148/radiol.12120216
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Filippi M, Riccitelli G, Mattioli F, Capra R, Stampatori C, Pagani E, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures – an explorative study. Radiology (2012) 262(3):932–40. doi:10.1148/radiol.11111299
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M. Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav (2012) 8(3):387–93. doi:10.1007/s11682-012-9160-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Cader S, Palace J, Matthews PM. Cholinergic agonism alters cognitive processing and enhances brain functional connectivity in patients with multiple sclerosis. J Psychopharmacol (2009) 23(6):686–96. doi:10.1177/0269881108093271
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Kooi EJ, Prins M, Bajic N, Beliën JA, Gerritsen WH, van Horssen J, et al. Cholinergic imbalance in the multiple sclerosis hippocampus. Acta Neuropathol (2011) 122(3):313–22. doi:10.1007/s00401-011-0849-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Geurts JJ, Wolswijk G, Bö L, van der Valk P, Polman CH, Troost D, et al. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain (2003) 126(Pt 8):1755–66. doi:10.1093/brain/awg179
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Wiebenga OT, Hulst HE, Kooi EJ, Killestein J, Geurts JJ. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology (2011) 77(22):1998–2000. doi:10.1212/WNL.0b013e318239c242
48. Strijbis EM, Inkster B, Vounou M, Naegelin Y, Kappos L, Radue EW, et al. Glutamate gene polymorphisms predict brain volumes in multiple sclerosis. Mult Scler (2013) 19(3):281–8. doi:10.1177/1352458512454345
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Filippi M, van den Heuvel MP, Fornito A, He Y, Hulshoff Pol HE, Agosta F, et al. Assessment of system dysfunction in the brain through MRI-based connectomics. Lancet Neurol (2013) 12(12):1189–99. doi:10.1016/S1474-4422(13)70144-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci (2009) 10(3):186–98. doi:10.1038/nrn2575
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Richiardi J, Gschwind M, Simioni S, Annoni JM, Greco B, Hagmann P, et al. Classifying minimally disabled multiple sclerosis patients from resting state functional connectivity. Neuroimage (2012) 62(3):2021–33. doi:10.1016/j.neuroimage.2012.05.078
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Schoonheim MM, Hulst HE, Landi D, Ciccarelli O, Roosendaal SD, Sanz-Arigita EJ, et al. Gender-related differences in functional connectivity in multiple sclerosis. Mult Scler (2012) 18(2):164–73. doi:10.1177/1352458511422245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Schoonheim MM, Geurts JJ, Landi D, Douw L, van der Meer ML, Vrenken H, et al. Functional connectivity changes in multiple sclerosis patients: a graph analytical study of MEG resting state data. Hum Brain Mapp (2013) 34(1):52–61. doi:10.1002/hbm.21424
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Van SJ, Gielen J, Laton J, D’hooghe MB, De KJ, Nagels G. Graph theoretical analysis indicates cognitive impairment in MS stems from neural disconnection. Neuroimage Clin (2014) 4:403–10. doi:10.1016/j.nicl.2014.01.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Rocca MA, Valsasina P, Meani A, Falini A, Comi G, Filippi M. Impaired functional integration in multiple sclerosis: a graph theory study. Brain Struct Funct (2014). doi:10.1007/s00429-014-0896-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Helekar SA, Shin JC, Mattson BJ, Bartley K, Stosic M, Saldana-King T, et al. Functional brain network changes associated with maintenance of cognitive function in multiple sclerosis. Front Hum Neurosci (2010) 4:219. doi:10.3389/fnhum.2010.00219
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Hardmeier M, Schoonheim MM, Geurts JJ, Hillebrand A, Polman CH, Barkhof F, et al. Cognitive dysfunction in early multiple sclerosis: altered centrality derived from resting-state functional connectivity using magneto-encephalography. PLoS One (2012) 7(7):e42087. doi:10.1371/journal.pone.0042087
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Schoonheim M, Geurts J, Wiebenga O, De Munck J, Polman C, Stam C, et al. Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Mult Scler (2013) 20(8):1058–65. doi:10.1177/1352458513516892
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Gamboa OL, Tagliazucchi E, von WF, Jurcoane A, Wahl M, Laufs H, et al. Working memory performance of early MS patients correlates inversely with modularity increases in resting state functional connectivity networks. Neuroimage (2014) 94:385–95. doi:10.1016/j.neuroimage.2013.12.008
60. Tewarie P, Hillebrand A, Schoonheim MM, van Dijk BW, Geurts JJ, Barkhof F, et al. Functional brain network analysis using minimum spanning trees in multiple sclerosis: an MEG source-space study. Neuroimage (2014) 88:308–18. doi:10.1016/j.neuroimage.2013.10.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: multiple sclerosis, cognition, connectivity, activation, networks, functional reorganization, functional MRI
Citation: Schoonheim MM, Meijer KA and Geurts JJG (2015) Network collapse and cognitive impairment in multiple sclerosis. Front. Neurol. 6:82. doi: 10.3389/fneur.2015.00082
Received: 06 February 2015; Paper pending published: 23 February 2015;
Accepted: 26 March 2015; Published online: 14 April 2015.
Edited by:
Maria Assunta Rocca, Università Vita-Salute San Raffaele, ItalyReviewed by:
Antonio Cerasa, Institute of Bioimaging and Molecular Physiology, ItalyMarisa Loitfelder, Medical University of Graz, Austria
Copyright: © 2015 Schoonheim, Meijer and Geurts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: m.schoonheim@vumc.nl
 Menno M. Schoonheim
Menno M. Schoonheim Kim A. Meijer
Kim A. Meijer Jeroen J. G. Geurts
Jeroen J. G. Geurts