- Department of Pediatrics and Interdisciplinary Program in Neuroscience, Georgetown University Medical Center, Washington, DC, USA
The large conductance, Ca2+-activated K+ channels (BKCa, KCa1.1) are expressed in various brain neurons where they play important roles in regulating action potential duration, firing frequency and neurotransmitter release. Membrane potential depolarization and rising levels of intracellular Ca2+ gated BKCa channels, which in turn results in an outward K+ flux that re/hyperpolarizes the membrane. The sensitivity of BKCa channels to Ca2+ provides an important negative-feedback system for Ca2+ entry into brain neurons and suppresses repetitive firing. Thus, BKCa channel loss-of-function gives rise to neuronal hyperexcitability, which can lead to seizures. Evidence also indicates that BKCa channels can facilitate high-frequency firing (gain-of-function) in some brain neurons. Interestingly, both gain-of-function and loss-of-function mutations of genes encoding for various BKCa channel subunits have been associated with the development of neuronal excitability disorders, such as seizure disorders. The role of BKCa channels in the etiology of some neurological diseases raises the possibility that these channels can be used as molecular targets to prevent and suppress disease phenotypes.
BkCa Channels and Neuronal Excitability
Intrinsic membrane properties play an important role in the control of neuronal activity in the central nervous system (CNS). Alterations of intrinsic membrane properties can contribute to diseases of neuronal excitability such as epilepsy. Potassium (K+) channels in particular are well known for their role in the regulation of membrane excitability due to their ability to stabilize the membrane potential. Compelling evidence indicates that K+ channels are critical molecular determinants for seizure generation and epileptogenesis. One particular type of K+ channel, the large conductance, Ca2+-activated K+ channel (BKCa, KCa1.1) is considered to be one of the intrinsic molecular determinants for the control of neuronal excitability in the CNS. Unlike other K+ channels, BKCa channels are activated by both voltage and elevated levels of intracellular Ca2+, resulting in large K+ conductances which in turn re/hyperpolarizes the membrane. The sensitivity of BKCa channels to Ca2+ provides an important negative feedback for Ca2+ entry into brain neurons; thus, BKCa channels may serve as a link between membrane depolarization and Ca2+ signaling to provide a rapid response to reduce or prevent neuronal hyperexcitability.
BKCa channels are tetramers of four α subunits, which form the ion channel pore, and four regulatory β (β 1–4) subunits that are expressed in various tissues, including the brain (Pallanek and Genetzky, 1994; Jiang et al., 1999). BKCa channels can also be regulated by acidification (Brelidze and Magleby, 2004; Hou et al., 2008), ethanol (Liu et al., 2008), protein kinase phosphorylation (Tian et al., 2001; Zhou et al., 2010), ubiquitination (Liu et al., 2014) and palmitoylation (Shipston, 2013; Zhou et al., 2012). Of particular importance, protein S-palmitoylation (or palmitoylation) and ubiquitination control the cell surface expression and activity of BKCa, thereby critically contributing to BKCa channel functions (Shipston, 2013; Liu et al., 2014). Notably, the palmitoylation of BKCa channel β subunits promotes the exit of the pore-forming α subunit from the endoplasmic reticulum and promotes BKCa channel surface expression (Chen et al., 2013). The BKCa channel α subunit is encoded by the Slo1 gene, which can be subjected to splicing to produce channels with different functional properties and sensitivity to Ca2+; including the STREX (stress-axis hormone-regulated exon) channels (Xie and McCobb, 1998; Chen et al., 2005). Expression profiling studies have reported that BKCa channel α subunits are broadly expressed in the CNS (Chang et al., 1997; Wanner et al., 1999; Sausbier et al., 2006). The regulatory BKCa channel β 1 and β 4 subunits are also expressed in the brain, whereas the β 2 and β 3 subunits are nearly absent in the brain (Tseng-Crank et al., 1996). BKCa channels are predominantly located at the axon and presynaptic terminals, associated with glutamatergic synapses in hippocampus and cortex and GABAergic synapses in the cerebellum (Knaus et al., 1996; Hu et al., 2001; Misonou et al., 2006; Martire et al., 2010). These channels are usually found in close proximity to N-methyl-D-asparte receptors (Isaacson and Murphy, 2001) and voltage-gated Ca2+ channels (CaV), including CaV1.2, CaV2.2, and CaV2.1 in the CNS (Marrion and Tavalin, 1998; Grunnet and Kaufmann, 2004). During an action potential (AP), both membrane depolarization and elevated intracellular Ca2+ can activate BKCa channels, which in turn contribute to AP fast repolarization, generate the fast component of the afterhyperpolarization (fAHP) and reduce Ca2+ influx via inactivation of CaV channels. Prominently, AP repolarization and fAHP significantly contribute to AP shape and duration. By controlling the AP shape and duration, BKCa channels can regulate neuronal excitability and some Ca2+ transients that underlie the release of neurotransmitter at presynaptic terminals.
The mechanisms underlying the inhibitory and excitatory role of BKCa channels are complex (Figure 1). Functional studies have reported that the activation of BKCa channels is hyperpolarizing; thus the resulting net effect on membrane excitability is inhibitory. However, evidence suggests that the activation of BKCa channels can also facilitate high-frequency firing in some brain neurons, including CA1 pyramidal cells of the hippocampus (Gu et al., 2007). In physiological conditions, BKCa channels activate slowly during an AP, allowing intracellular Ca2+ to activate Ca2+-dependent conductances such as the small conductance Ca2+-activated K+ (SKCa) channels, thereby inhibiting repetitive firing. The inhibitory effect following the activation of BKCa channels may result from a delay in the development of an AP spike or decrease in fAHP conductances. Altered extracellular K+ levels can modify the cell membrane potential to persistently depolarized values that may lead to paroxysmal discharges (Lebovitz, 1996). Interestingly, conversion from regular firing into burst firing upon the elevation of extracellular K+ has been observed in hippocampal slices (Jensen et al., 1994; Jensen and Yaari, 1997). Blockade of BKCa channels also can inhibit neuronal firing because the resulting AP broadening can allow the activation of slow-onset voltage-gated K+ channels, such as small SKCa channels and delayed rectifier K+ channels. The resulting K+ currents associated with an increased inactivation of voltage-gated Na+ (NaV) channels could slow the depolarization during an interspike interval. Further, excitation following the activation of upregulated BKCa channels may result from their role in the generation of fast spike repolarization and fAHP, which would favor a reduced activation of SKCa channels and delayed rectifier K+ channels and would indirectly facilitate the recovery of NaV from inactivation (Gu et al., 2007). The upregulation of BKCa channels may cause large increase in extracellular K+, which in turn reduces the driving force for inhibitory K+ currents leading to enhanced neuronal excitability. The activation of BKCa channels can reduce neurotransmitter (GABA) release by shortening the duration of depolarization to allow Ca2+ entry via CaV channels, resulting in enhanced neuronal excitability (Hu et al., 2001; Raffaelli et al., 2004). There is also a possibility that the inhibitory and excitatory action of BKCa channels may be age dependent. Indeed, smaller BKCa channel currents were recorded in pyramidal neurons of the prefrontal cortex in developing animals compared with adolescent and adult animals (Ksiazek et al., 2013). Multiple lines of evidence indicate that a lower availability and/or expression of BKCa channels may contribute to the broadening of APs during repetitive firing (Shao et al., 1999; Faber and Sah, 2003). Therefore, the lower availability of BKCa channels in young animals may facilitate neuronal activity during this developmental stage. Given the relevance of BKCa channels in the control of neuronal excitability, these channels have been implicated in the pathophysiology of several neurological disorders associated with altered neuronal excitability, including seizure disorders.
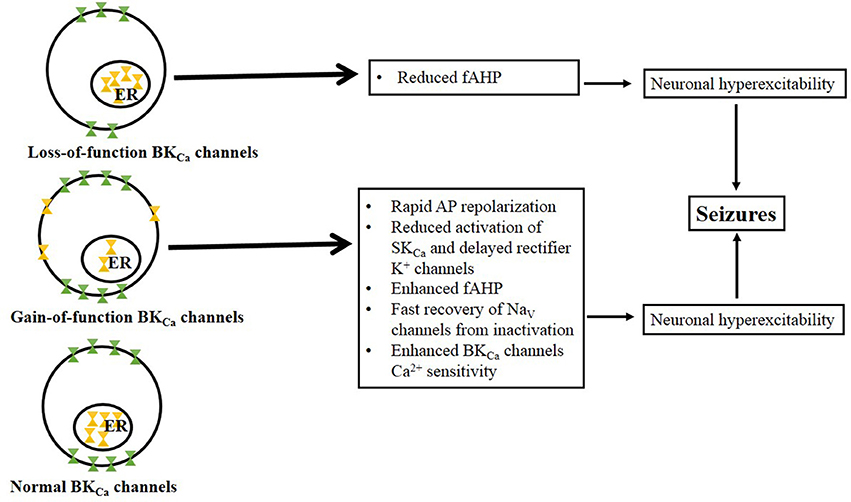
Figure 1. Proposed mechanisms associated with BKCa loss-of-function and gain-of-function channels. BKCa channel loss-of-function occurs when there is low abundance of the channel at the membrane surface but no change in the BKCa channel number in the endoplasmic reticulum (ER, note that ubiquitination prevent channels from trafficking to the cell surface). Potential mechanisms underlying neuronal hyperexcitability following BKCa channels loss-of-function include reduced fAHP conductances. BKCa channel gain-of-function is characterized by the release of ubiquitinated BKCa channels from the ER and their insertion into the membrane surface (Liu et al., 2014). Thus, impairing ubiquitination may lead to overexpression of BKCa channels relative to control conditions. Potential mechanisms underlying neuronal hyperexcitability following BKCa channels gain-of-function include: rapid AP repolarization that would favor reduced activation of SKCa and delayed rectifier K+ channels as well as facilitated the rate of recovery of NaV channels from inactivation.
BkCa Channel Loss-of-Function Hypothesis
BkCa Channel Loss-of-Function and Enhanced Neuronal Excitability in Seizure Disorders
Epilepsy consists of a group of chronic neurological disorders characterized by spontaneous and recurrent seizures. These seizures result from aberrant neuronal excitability associated with abnormal connections in the brain. Because the activation of BKCa channels limits the depolarization-induced bursting activity in neurons, it is assumed that a loss-of-function in BKCa channels will promote neuronal hyperexcitability, which can lead to seizures. Accordingly, reduced fAHP conductances were found in dentate gyrus granule cells obtained from patients suffering from temporal lobe epilepsy (Williamson et al., 1993). Similarly, idiopathic generalized epilepsy (mostly typical absence epilepsy) in humans has been associated with a single nucleotide deletion in exon 4 (delA750) of the KCNMB3 gene encoding for BKCachannel β 3 subunit (Lorenz et al., 2007). When expressed in a heterologous system, this mutation (BKCa channel β 3b-V4 subunit isoform) exhibited BKCa channel loss-of-function, characterized by fast inactivation kinetics (Hu et al., 2003). The mutated KCNMB3 gene also has been found in patients with dup(3q) syndrome with seizures (Riazi et al., 1999).
BKCa channel loss-of-function has also been implicated in the pathophysiology of animal models of seizures and epilepsy. A transient loss of fAHP conductances was found in subicular neurons following a kindling model of epileptogenesis (Behr et al., 2000). In the genetically epilepsy-prone rat (GEPR), an inherited model of generalized tonic-clonic epilepsy, reduced fAHP conductances were reported in CA3 neurons of the hippocampus (Verma-Ahuja et al., 1995). Similarly, in preliminary experiments, we found that the current density of BKCa channels is significantly reduced in inferior colliculus (IC) neurons, the site of seizure initiation in this model. However, no significant change was observed in the abundance of BKCa channel α subunit proteins in IC neurons of the GEPR (N'Gouemo et al., 2009). Similarly, the expression of BKCa channel α subunit was not altered in the dentate gyrus of the Krushinskii-Molodkina rat, a model of inherited epilepsy (Savina et al., 2014). Nevertheless, the protein expression of BKCa channel β 4 subunits was elevated in the dentate gyrus of the Krushinskii-Moslodkina rat (Savina et al., 2014). The upregulation of β 4 subunit is consistent with loss-of-function because this subunit inhibits BKCa channel activity (Brenner et al., 2005). In a model of alcohol withdrawal seizures, BKCa channel loss-of-function was reported and characterized by reduced current density, decreased channel conductance and lower protein abundance of BKCa channel α subunit in IC neurons (N'Gouemo and Morad, 2014). However, these changes outlasted the finite period of alcohol withdrawal seizure susceptibility, suggesting that BKCa channel loss-of-function in IC neurons was associated with the long-term effects of alcohol withdrawal hyperexcitability. Whether BKCa channels in IC neurons play an important role in the pathogenesis of alcohol withdrawal seizures remains to be determined. In a pilocarpine post-status epilepticus model, a downregulation of BKCa channel α subunit mRNA and protein was found in the cortex and hippocampus, consistent with a loss-of-function of BKCa channels associated with seizure generation (Pacheco Otalora et al., 2008; Ermolinsky et al., 2011). Further analysis revealed that the remaining BKCa channels in the dentate gurus were essentially made of the BKCa channel STREX splice variant instead of the ZERO variant (Ermolinsky et al., 2011). Interestingly, inserting the STREX splice variant shifts the conductance/voltage relation of BKCa channels to the left so that the channels are active at more physiological Ca2+ and voltage levels (Shipston, 2013). However, elevated intracellular Ca2+ is associated with seizure activity and epileptogenesis (Sanabria et al., 2001; Raza et al., 2004), suggesting an altered function of the remaining STREX BKCa channels in the pilocarpine model.
BkCa Channel Loss-of-Function and Enhanced Neuronal Excitability in Autism Spectrum Disorders
Autism spectrum disorders (ASD) are a heterogeneous group of genetic neurodevelopmental disorders characterized by impairment of social communication and behavioral problems. Interestingly, studies have reported a co-occurrence of ASD and epilepsy (Deykin and MacMahon, 1979). The prevalence of epilepsy and associated electroencephalogram abnormalities in ASD significantly exceeded that of the normal population (Tuchman and Rapin, 1997). The higher incidence of epileptiform electroencephalogram abnormalities was also reported in children with ASD without epilepsy (Tuchman and Rapin, 1997). Thus, autism may be classified as a disorder of neuronal excitability, suggesting a potential role for ion channels in the etiology of ASD. ASD-linked ion channels of interest include BKCa channels. A mutation in the KCNAM1 gene, which encodes for the α subunit of BKCa channels, has been reported in some ASD patients with epilepsy (Laumonnier et al., 2006). The mutated KCNAM1 gene also causes haploinsufficiency in ASD patients, suggesting a potential role of BKCa channels in the pathogenesis of ASD (Laumonnier et al., 2006). When expressed in a heterologous system, this mutation exhibits reduced BKCa channel currents consistent with a loss-of-function (Laumonnier et al., 2006). Whether the downregulation of BKCa channels directly contributes to the pathogenesis of autism-epilepsy phenotype remains unknown.
BkCa Channel Loss-of-Function and Reduced Neuronal Excitability in Seizure Disorders
Evidence shows that pharmacological blockade of BKCa channels can trigger seizures and status epilepticus, providing compelling evidence that BKCa channel loss-of-function can contribute to epileptogenesis (Young et al., 2003). However, mice lacking BKCa channel α (and β 1) subunits do not exhibit spontaneous seizures, consistent with no change or reduced CNS excitability (Sausbier et al., 2004). Thus, the elevated seizure susceptibility observed in animal models cannot be explained solely by a downregulation of BKCa channel α subunits. Notably, evidence shows that BKCa channels can be subjected to ubiquitination by CRL4ACRBN and are therefore retained in the endoplasmic reticulum and prevented from trafficking to the cell surface. Deregulation of this control mechanism results in enhanced activity of neuronal BKCa channels and epileptogenesis (Liu et al., 2014). Notably, the cereblon (CRBN) co-localizes with BKCa channels in brain neurons and regulate their surface expression (Jo et al., 2005). The CRBN gene is highly expressed in the hippocampus, consistent with its role in the pathogenesis of limbic seizures (Liu et al., 2014).
BkCa Channel Gain-of-Function Hypothesis
BkCa Channel Gain-of-Function and Enhanced Neuronal Excitability in Seizure Disorders
Although BKCa channels are thought to reduce neuronal firing, evidence indicates that the gain-of-function of these channels can contribute to bursting activity and epileptogenesis. Indeed, upregulation of the α subunit and downregulation of the β 4 subunit of BKCa channels were found in the dentate gyrus neurons of Krushinskii-Molodkin rats subjected to audiogenic kindling, which induced enhanced seizure severity (Savina et al., 2014). These findings are consistent with the BKCa channel gain-of-function associated with enhanced seizure severity because the β 4 subunit inhibits BKCa channel activity. Notably, genetic deletion of the β 4 subunit of BKCa channels facilitates the development of pilocarpine-induced seizures that are associated with gain-of-function of BKCa channels, as characterized by elevated cell-surface expression of BKCa channels, enhanced Ca2+ sensitivity to BKCa channels, larger currents and high-frequency firing in the dentate gyrus of the hippocampus (Brenner et al., 2005; Shruti et al., 2012).
BKCa channel gain-of-function has also been found in human epilepsy. Accordingly, in a family of patients suffering from generalized epilepsy (mostly absence epilepsy) and paroxysmal dyskinesia, a missense mutation (D434G) in exon 10 of the KCNMA1 gene that encodes the BKCa channel α subunit has been found (Du et al., 2005). When expressed in a heterologous system, this mutation gave rise to gain-of-function of BKCa channel currents characterized by larger currents, elevated open channel probability and enhanced Ca2+ sensitivity to BKCa channels (Du et al., 2005; Wang et al., 2009; Yang et al., 2010). The D434G mutation gain-of-function was potentiated in the presence of β 1, β 2, and β 4 subunits of BKCa channels (Díez-Sampedro et al., 2006; Lee and Cui, 2009). Notably, a polymorphism in the β 4 subunit has been associated with human epilepsy (Cavalleri et al., 2007). These findings suggest that D434G mutation-induced changes in BKCa channels contribute to neuronal hyperexcitability and lead to generalized seizures and paroxysmal dyskinesia.
BkCa Channel Gain-of-Function and Reduced Neuronal Excitability in Seizure Disorders
BKCa channels are found in excitatory neurons located in several brain sites, including the hippocampus, where they may promote high-frequency firing (Gu et al., 2007). Blockade of BKCa channels in these brain sites may reduce or suppress neuronal hyperexcitability. Consistent with this hypothesis, the blockade of BKCa channels suppressed pentylenetetrazole-induced epileptiform activity as well as spontaneous bursting activity in cortical neurons obtained from EL mouse, an inherited model of epilepsy (Jin et al., 2000). Similarly, picrotoxin-induced generalized tonic-clonic seizures give rise to BKCa channel gain-of-function characterized by elevated currents and high-frequency firing in somatosensory (barrel) cortical neurons of pre-sensitized animals (Shruti et al., 2008). Accordingly, the blockade of BKCa channels suppressed these picrotoxin-induced generalized tonic-clonic seizures (Sheehan et al., 2009). Thus, picrotoxin-induced seizure pre-sensitization may cause a maladaptive regulation (e.g., exit from the endoplasmic reticulum) of BKCa channels in brain neurons. In a fly model of ethanol intoxication/withdrawal, a blockade of Slo1 gene neural promoter prevented the occurrence of ethanol-induced enhancement of electrographical seizure susceptibility, suggesting BKCa channel gain-of-function in the pathogenesis of alcohol withdrawal seizures (Ghezzi et al., 2012). However, this report raises some controversy with a rodent model of alcohol withdrawal seizures (N'Gouemo and Morad, 2014).
Conclusion
The role of BKCa channels in the pathophysiology of diseases of neuronal excitability is complex, in part because the activity of these channels can be regulated by many metabolic factors that alter neuronal excitability, including phosphorylation and acidification. Compelling evidence suggests that BKCa channel loss-of-function and gain-of-function can both contribute to neuronal hyperexcitability that leads to enhanced seizure susceptibility. The identification of BKCa channel subunit mutations has been critical in determining the role of these channels in etiology and mechanisms for epileptogenesis and seizure generation, raising the possibility that BKCa channels may represent potential molecular targets for seizure suppression.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the NIH Public Health Service Grant AA020073. The author would like to thank Dr. Gholam Motamedi for helpful discussions and critical reading.
References
Behr, J., Gloveli, T., and Heinemann, U. (2000). Kindling induces a transient suppression of afterhyperpolarization in rat subicular neurons. Brain Res. 867, 259–264. doi: 10.1016/S0006-8993(00)02324-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brelidze, T. I., and Magleby, K. L. (2004). Protons block BK channels by competitive inhibition with K+ and contribute to the limits of unitary currents at high voltages. J. Gen. Physiol. 123, 305–319. doi: 10.1085/jgp.200308951
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brenner, R., Chen, Q. H., Vilaythong, A., Toney, G. M., Noebels, J. L., and Aldrich, R. W. (2005). BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 28, 1752–1759. doi: 10.1038/nn1573
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cavalleri, G. L., Weale, M. E., and Shianna, K. V. (2007). Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case-control study. Lancet Neurol. 6, 970–980. doi: 10.1016/S1474-4422(07)70247-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chang, C. P., Dworetzky, S. I., Wang, J., and Goldstein, M. E. (1997). Differential expression of the alpha and beta subunits of the large-conductance calcium-activated potassium channel; implication for channel diversity. Brain Res. Mol. Brain Res. 45, 33–40. doi: 10.1016/S0169-328X(96)00230-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, L., Bi, D., Tian, L., McClafferty, H., Steeb, F., Ruth, P., et al. (2013). Palmitoylation of the β 4-subunit regulates surface expression of large conductance calcium-activated potassiumchannel splice variants. J. Biol. Chem. 288, 13136–13144. doi: 10.1074/jbc.M113.461830
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, L., Tian, L., MacDonald, S. H., McClafferty, H., Hammond, M. S., Huibant, J. M., et al. (2005). Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J. Biol. Chem. 280, 33599–33609. doi: 10.1074/jbc.M505383200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Deykin, E. Y., and MacMahon, B. (1979). The incidence of seizures among children with autistic symptoms. Am. J. Psychiatry 136, 1310–1312.
Díez-Sampedro, A., Silverman, W. R., Bautista, J. F., and Richerson, G. B. (2006). Mechanism of increased open probability by a mutation of the BK channel. J. Neurophysiol. 96, 1507–1516. doi: 10.1152/jn.00461.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Du, W., Bautista, J. F., Yang, H., Diez-Sampedro, A., You, S. A., Wang, L., et al. (2005). Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37, 733–738. doi: 10.1038/ng1585
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ermolinsky, B., Skinner, F., Garcia, I., Arshadmansab, M. F., Otalora, L. F., Zarei, M. M., et al. (2011). Upregulation of STREX splice variant of the large conductance Ca2+-activated potassium (BK) channel in a rat model of mesial temporal lobe epilepsy. Neurosci. Res. 69, 73–80. doi: 10.1016/j.neures.2010.09.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Faber, E. S., and Sah, P. (2003). Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J. Physiol. 552(Pt 2), 483–497. doi: 10.1113/jphysiol.2003.050120
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ghezzi, A., Krishnan, H. R., and Atkinson, N. S. (2012). Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addict. Biol. doi: 10.1111/j.1369-1600.2012.00465.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grunnet, M., and Kaufmann, W. A. (2004). Coassembly of big conductance Ca2+-activated K+ channels and L-type voltage-gated Ca2+ channels in rat brain. J. Biol. Chem. 279, 36445–36453.
Gu, N., Vervaeke, K., and Storm, J. F. (2007). BK potassium channels facilitate high-frequency firing and cause early frequency adaptation in rat CA1 hippocampal pyramidal cells. J. Physiol. 580, 859–882. doi: 10.1113/jphysiol.2006.126367
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hou, S., Xu, R., Heinemann, S. H., and Hoshi, T. (2008). Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat. Struct. Mol. Biol. 15, 403–410. doi: 10.1038/nsmb.1398
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hu, H., Shao, L. R., Chavoshy, S., Gu, N., Trieb, M., Behrens, R., et al. (2001). Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J. Neurosci. 21, 9585–9597.
Hu, S., Labuda, M. Z., Pandolfo, M., Goss, G. G., McDermid, H. E., and Ali, D. W. (2003). Variants of the KCNMB3 regulatory subunit of maxi BK channels affect channel inactivation. Physiol. Genomics 15, 191–198.
Isaacson, J. S., and Murphy, G. J. (2001). Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca2+-activated K+ channels. Neuron 31, 1027–1034. doi: 10.1016/S0896-6273(01)00428-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jensen, M. S., Azouz, R., and Yaari, Y. (1994). Variant firing patterns in rat hippocampal pyramidal cells modulated by extracellular potassium. J. Neurophysiol. 71, 831–839.
Jensen, M. S., and Yaari, Y. (1997). Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. J. Neurophysiol. 77, 1224–1233.
Jiang, Z., Wallner, M., Meera, P., and Toro, L. (1999). Human and rodent MaxiK channel beta-subunit genes: cloning and characterization. Genomics 55, 57–67. doi: 10.1006/geno.1998.5627
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jin, W., Sugaya, A., Tsuda, T., Ohguchi, H., and Sugaya, E. (2000). Relationship between large conductance calcium-activated potassium channel and bursting activity. Brain Res. 860, 21–28. doi: 10.1016/S0006-8993(00)01943-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jo, S., Lee, K.-H., Song, S., Jung, Y.-K., and Park, C.-S. (2005). Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channel in rat brain. J. Neurochem. 94, 1212–1224. doi: 10.1111/j.1471-4159.2010.06938.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Knaus, H. G., Schwarzer, C., Koch, R. O., Eberhart, A., Kaczorowski, J. G., Glossmann, H., et al. (1996). Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J. Neurosci. 16, 955–963.
Ksiazek, A., Ladno, W., Szulczyk, B., Grzelka, K., and Szulczyk, P. (2013). Properties of BK-type Ca++-dependent K+ channel currents in medial prefrontal cortex pyramidal neurons in rats of different ages. Front. Cell. Neurosci. 7:185. doi: 10.3389/fncel.2013.00185
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Laumonnier, F., Roger, S., Guérin, P., Molinari, F., M'rad, R., Cahard, D., et al. (2006). Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am. J. Psychiatry 163, 1622–1629.
Lebovitz, R. M. (1996). Quantitative examination of dynamic interneuronal coupling via single-spike extracellular potassium ion transients. J. Theor. Biol. 180, 11–25.
Lee, U. S., and Cui, J. (2009). β-subunit-specific modulations of BK channels function by a mutation associated with epilepsy and dyskinesia. J. Physiol. 587, 1481–1498. doi: 10.1113/jphysiol.2009.169243
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, J., Vaithianathan, T., Manivannan, K., Parrill, A., and Dopico, A. M. (2008). Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol. Pharmacol. 74, 628–640. doi: 10.1124/mol.108.048694
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, J., Ye, J., Zou, X., Xu, Z., Feng, Y., Zou, X., et al. (2014). CRL4A(CRBN) E3 ubiquitin ligase restricts BK channel activity and prevents epileptogenesis. Nat. Commun. 5, 3924. doi: 10.1038/ncomms4924
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lorenz, S., Heils, A., Kasper, J. M., and Sander, T. (2007). Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. Am. J. Med. Genet. 144B, 10–13. doi: 10.1002/ajmg.b.30369
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marrion, N. V., and Tavalin, S. J. (1998). Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature 395, 900–905.
Martire, M., Barrese, V., D'Amico, M., Iannotti, F. A., Pizzarelli, R., Samengo, I., et al. (2010). Pre-synaptic BK channels selectively control glutamate versus GABA release from cortical and hippocampal nerve terminals. J. Neurochem. 115, 411–422. doi: 10.1111/j.1471-4159.2010.06938.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Misonou, H., Menegola, M., Buchwalder, L., Park, E. W., Meredith, A., Rhodes, K. J., et al. (2006). Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J. Comp. Neurol. 496, 289–302. doi: 10.1002/cne.20931
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
N'Gouemo, P., and Morad, M. (2014). Alcohol withdrawal is associated with a downregulation of large-conductance Ca2+-activated K+ channels in rat inferior colliculus neurons. Psychopharmacology 231, 2009–2018. doi: 10.1007/s00213-013-3346-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
N'Gouemo, P., Yasuda, R. P., and Faingold, C. L. (2009). Protein expression of small conductance calcium-activated potassium channels is altered in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Res. 1270, 107–111. doi: 10.1016/j.brainres.2009.02.034
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pacheco Otalora, L. F., Hernandez, E. F., Arshadmansab, M. F., Francisco, S., Willis, M., Ermolinsky, B., et al. (2008). Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res. 1200, 116–131. doi: 10.1016/j.brainres.2008.01.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pallanek, L., and Genetzky, B. (1994). Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channels gene slowpoke. Hum. Mol. Genet. 3, 1239–1243. doi: 10.1093/hmg/3.8.1239
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Raffaelli, G., Saviane, C., Mohajerani, M. H., Perazani, P., and Cherubini, E. (2004). BK potassium channels control transmitter release at CA3-CA3 synapses in rat hippocampus. J. Physiol. 557, 147–157. doi: 10.1113/jphysiol.2004.062661
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Raza, M., Blair, R. E., Sombati, S., Carter, D. S., Deshpande, L. S., and DeLorenzo, R. J. (2004). Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl. Acad. Sci. U.S.A. 101, 17522–17527. doi: 10.1073/pnas.0408155101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Riazi, M. A., Brinkman-Mills, P., Jonhson, A., Naylor, S. L., Minoshima, S., Shimizu, N., et al. (1999). Identification of a putative regulatory subunit of a calcium-activated potassium channel in the dup(3q) syndrome region and a related sequence on 22q11.2. Genomics 62, 90–94. doi: 10.1006/geno.1999.5975
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sanabria, E. R. G., Su, H., and Yaari, Y. (2001). Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J. Physiol. 532, 205–216. doi: 10.1111/j.1469-7793.2001.0205g.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sausbier, M., Hu, H., Arntz, C., Feil, S., Kamm, S., Adelsberger, H., et al. (2004). Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc. Natl. Acad. Sci. U.S.A. 101, 9474–9478. doi: 10.1073/pnas.0401702101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sausbier, U., Sausbier, M., Sailer, C. A., Arntz, C., Kauss, H. G., Neuhuber, W., et al. (2006). Ca2+ -activated K+ channels of the BK-type in the mouse brain. Histochem. Cell. Biol. 125, 725–741. doi: 10.1007/s00418-005-0124-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Savina, T. A., Levin, S. G., Poletaeva, I. I., Fedotova, I. B., and Shchipakina, T. G. (2014). Audiogenic kindling changes the subunit composition of BK-channels in dentate gyrus of Krushinskii-Molodkina rats. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 8, 111–115. doi: 10.1134/S1990747813050164
Shao, L. R., Halvorsrud, R., Borg-Graham, L., and Storm, J. F. (1999). The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. 521, 135–146. doi: 10.1111/j.1469-7793.1999.00135.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sheehan, J. J., Benedetti, B. L., and Barth, A. L. (2009). Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia 50, 711–720. doi: 10.1111/j.1528-1167.2008.01888.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shipston, M. J. (2013). Regulation of large conductance calcium- and voltage-activated potassium (BK) channels by S-palmitoylation. Biochem. Soc. Trans. 41, 67–71. doi: 10.1042/BST20120226
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shruti, S., Urban-Ciecko, J., Fitzpatrick, J. A., Brenner, R., Bruchez, M. P., and Barth, A. L. (2012). The brain-specific beta4 subunit downregulates BK channel cell surface expression. PLoS ONE 7:e33429. doi: 10.1371/journal.pone.0033429
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shruti, S., Clem, R. L., and Barth, A. L. (2008). A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiol. Dis. 30, 323–330. doi: 10.1016/j.nbd.2008.02.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tian, L., Duncan, R. R., Hammond, M. S., Coghill, L. S., Wen, H., Rusinova, R., et al. (2001). Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J. Biol. Chem. 276, 7717–7720. doi: 10.1074/jbc.C000741200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tseng-Crank, J., Godinot, N., Johansen, T. E., Ahring, P. K., Strobaek, D., Mertz, R., et al. (1996). Cloning, expression, and distribution of a Ca2+-activated K+ channel beta-subunit from human brain. Proc. Natl. Acad. Sci. U.S.A. 93, 9200–9205. doi: 10.1073/pnas.93.17.9200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tuchman, R. F., and Rapin, I. (1997). Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics 99, 560–566.
Verma-Ahuja, S., Evans, M. S., and Espinosa, J. A. (1995). Evidence of increased excitability in GEPR hippocampus preceding development of seizure susceptibility. Epilepsy Res. 31, 161–173. doi: 10.1016/S0920-1211(98)00027-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wang, B., Rothberg, B. S., and Brenner, R. (2009). Mechanism of increased BK channel activation from channel mutation that causes epilepsy. J. Gen. Physiol. 133, 283–294. doi: 10.1085/jgp.200810141
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wanner, S. G., Koch, R. O., Koschak, A., Trieb, M., Garcia, M. L., Kaczorowski, G. J., et al. (1999). High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution and subunit composition. Biochemistry 38, 5392–5400. doi: 10.1021/bi983040c
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Williamson, A., Spencer, D. D., and Shepherd, G. N. (1993). Comparison between membrane and synaptic properties of the human and rodent granule cells. Brain Res. 622, 194–202. doi: 10.1016/0006-8993(93)90819-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xie, J., and McCobb, D. P. (1998). Control of alternative splicing of potassium channels by stress hormones. Science 280, 443–446.
Yang, J., Krishnamoorthy, G., Saxena, A., Zhang, G., Shi, J., Yang, H., et al. (2010). An epilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+ sensing. Neuron 66, 871–883. doi: 10.1016/j.neuron.2010.05.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Young, K. L., Villar, D., Carson, T. L., Ierman, P. M., Moore, R. A., and Bottoff, M. R. (2003). Tremorgenic mycotoxin intoxication with penitrem A and roquefortine in two dogs. J. Am. Vet. Med. Assoc. 222, 52–53. doi: 10.2460/javma.2003.222.52
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhou, X.-B., Wulfsen, I., Utku, E., Sausbier, M., Wieland, T., Ruth, P., et al. (2010). Dual role of protein kinase C on BK channels regulation. Proc. Natl. Acad. Sci. U.S.A. 107, 8005–8010. doi: 10.1073/pnas.0912029107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhou, X., Wulfsen, I., Korth, M., McClafferty, H., Lukowski, R., Shipston, M. J., et al. (2012). Palmitoylation and membrane association of the stress axis regulated insert (STREX) controls BK channel regulation by protein kinase C. J. Biol. Chem. 287, 32161–32171. doi: 10.1074/jbc.M112.386359
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: autism, alcohol withdrawal seizures, epilepsy, gain-of-function, loss-of-function
Citation: N'Gouemo P (2014) BKCa channel dysfunction in neurological diseases. Front. Physiol. 5:373. doi: 10.3389/fphys.2014.00373
Received: 15 July 2014; Accepted: 10 September 2014;
Published online: 29 September 2014.
Edited by:
Thomas M. Weiger, University of Salzburg, AustriaReviewed by:
Brad S. Rothberg, Temple University School of Medicine, USAJose Bargas, Universidad Nacional Autónoma de México, Mexico
Copyright © 2014 N'Gouemo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prosper N'Gouemo, Department of Pediatrics and Interdisciplinary Program in Neuroscience, Georgetown University Medical Center, 3900 Reservoir Rd, NW, Washington, DC 20057, USA e-mail: pn@georgetown.edu
 Prosper N'Gouemo
Prosper N'Gouemo