Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice
1
Laboratory of Behavioral Neuroscience, National Institute of Mental Health, USA
2
Department of Cell Biology and Neurosciences, Istituto Superiore di Sanita, Italy
3
Section on Neural Gene Expression, NIMH, USA
4
Department of Psychology, Kenyon College, USA
Mice are a nocturnal species, whose social behaviors occur primarily during the dark phase of the circadian cycle. However, laboratory rodents are frequently tested during their light phase, for practical reasons. We investigated the question of whether light phase testing presents a methodological pitfall for investigating mouse social approach behaviors. Three lines of mice were systematically compared. One cohort of each line was raised in a conventional lighting schedule and tested during the light phase, under white light illumination; another cohort was raised in a reverse lighting schedule and tested during their dark phase, under dim red light. Male C57BL/6J (B6) displayed high levels of sociability in our three-chambered automated social approach task when tested in either phase. BTBR T+ tf/J (BTBR) displayed low levels of sociability in either phase. Five cohorts of vasopressin receptor subtype 1b (Avpr1b) null mutants, heterozygotes, and wildtype littermate controls were tested in the same social approach paradigm: three in the dark phase and two in the light phase. All three genotypes displayed normal sociability in four out of the five replications. In the juvenile play test, testing phase had no effect on play soliciting behaviors in Avpr1b mice, but had modest effects on nose sniff and huddling. Taken together, these findings indicate that testing phase is not a crucial factor for studying some forms of social approach in juvenile and adult mice.
Mouse behaviors vary across the light∕dark cycle (Kopp, 2001
; Refinetti, 2006
). In mammals, ambient light is the best known factor that regulates circadian timing, primarily through neurons in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus (Menaker et al., 1997
; Redlin and Mrosovsky, 2004
; Reppert and Weaver, 2002
). Non-photic environmental cues such as social milieu, sights, sounds, smell, and other habitat factors also contribute to circadian variations in behaviors (Mistlberger and Skene, 2004
; Zisapel et al., 1999
). Findings obtained from several species suggest that the ability to adjust behavioral activities according to non-photic factors offers adaptive advantages, such as reducing predation risk and conspecific competitions (Daily and Ehrlich, 1996
; Mistlberger and Skene, 2004
; Mrosovsky, 2003
).
Inbred strains of mice maintained for laboratory research are mostly derived from several sub-species of Mus musculus (Petkov et al., 2004
; Silver, 1995
). In the wild, Mus musculus are generally nocturnal, displaying most of their activity during the night (McLennan and Taylor-Jeffs, 2004
; Refinetti, 2004
; Whishaw et al., 1999
). However, in practice, it is common for research laboratories to conduct mouse behavioral experiments during the light phase. Although this approach has been criticized as ethologically incorrect (Beeler et al., 2006
; Hossain et al., 2004
; Jennings et al., 1998
; Roedel et al., 2006
), existing data on effects of testing phase and lighting conditions on mouse behaviors are limited. Roedel et al. (2006)
found that, in a modified hole board test, DBA mice tested in the light phase showed lower levels of exploratory activities and risk assessment than those tested in the dark phase. The same study also demonstrated that cognitive performance was compromised in mice tested in the light phase. Kelliher et al. (2000)
found that Sprague–Dawley rats tested in the dark phase exhibited lower levels of escape behaviors in the forced swim test compared to those tested in the light phase. Hossain et al. (2004)
found that strain differences (129S1∕SvImJ, B6, and their F1 offspring) in the open field test were more detectable in the dark phase, and strain differences in the tail flick test were detectable only in the light phase. Rats and mice tend to exhibit lower levels of social interactions in a brightly lit versus dimly lit open field arena, reflecting higher levels of anxiety-like behaviors in the former condition (File and Seth, 2003
). Other reports indicate that testing phase does not have a significant impact on behavioral tests. Circadian testing phase did not affect the outcomes of a social interaction test and a social recognition test in mice (Hossain et al., 2004
). In a study comparing B6 and 129S1∕SvImJ mice tested in opposite circadian cycles, Beeler et al. (2006)
found no significant effect of testing phase on a number of commonly used behavioral paradigms including open field, elevated plus-maze, Morris water maze, novel object exploration, and motor response to amphetamine. In sum, it remains unclear to what extent circadian phase influences the scores obtained when mice are tested on various behavioral paradigms designated to evaluate emotional responses, cognitive performances, and social behaviors.
Our laboratory has begun to characterize social approach behaviors in transgenic, knockout, and inbred strains of mice, toward establishing relevant mouse models for human disorders defined by profound deficits in social interactions, such as autism. Mice tend to engage in high levels of social interaction in the dark phase (Arakawa et al., 2007
; Laviola et al., 1994
; Panksepp and Lahvis, 2006
; Terranova et al., 1998
), raising the question of whether light phase testing is appropriate for studying social approach behaviors in laboratory mice. Existing data regarding this issue are few (Hossain et al., 2004
; Paterson and Vickers, 1984
), and not sufficient to provide clear guidance.
The main goal of the present experiments was to compare scores on social behavior tests conducted during the light phase, under white light illumination, versus those conducted during the dark phase, under dim red light illumination, in two inbred strains and three genotypes of a line of neuropeptide receptor mutant mice. C57BL∕6J (B6) and BTBR T+ tf∕J (BTBR) are two inbred strains with distinctly contrasting social behavior phenotypes (Bolivar et al., 2007
; McFarlane et al., 2007
; Moy et al., 2007
). Vasopressin is a hypothalamic neuropeptide that has been shown to mediate social recognition, sexual, parental, and aggressive interactions (Ferris, 2005
; Hammock and Young, 2006
; Nair and Young, 2006
; Wang et al., 1998
). Null mutation of the vasopressin receptor subtype 1B gene (Avpr1b) produces deficits in social recognition and reduced aggression in male mice (Wersinger et al., 2002
, 2006
). A second goal of this study is to address the related question of replicability of mouse behavioral findings (Crabbe et al., 1999
; Wahlsten et al., 2003
) across circadian conditions. We conducted social approach testing with five independent cohorts of Avpr1b null mutants, heterozygotes, and wildtype littermate controls, two during the light phase and three during the dark phase. Finally, effects of circadian phase on juvenile play behaviors were evaluated in Avpr1b mice.
Animals
All breeding, housing, and behavioral testing was conducted in strict compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee. Mice were weaned at postnatal day 21, then group housed by gender in standard mouse cages containing 2–4 mice per cage. For the Avpr1b experiments, each home cage consisted of 2–4 littermate mice of mixed genotypes. Cages were housed in ventilated racks in temperature (20°C) and humidity (∼55%) controlled colony rooms. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. The two colony rooms used for breeding and housing subjects were both on 12:12 light∕dark cycles, one with lights on from 6:00 AM to 6:00 PM, and the other with lights on from 9:00 PM to 9:00 AM. Animal care staff entered the reverse light cycle room only before 9 AM. Red lights were turned on if staff or experimenters need to enter the room during the dark cycle. All experiments were conducted between 10:00 AM and 4:00 PM. Light phase testing was conducted under two 75-watt fluorescence bulb desk lamps. Dark phase testing was conducted under the illumination of a single 25-watt incandescent red light bulb desk lamp. Red light was used to simulate darkness, since murine eyes are insensitive to red light (McLennan and Taylor-Jeffs, 2004
). At the beginning of each test day, light intensity was tested and adjusted to achieve lighting homogeneity in and around the apparatus. The light level was approximately 2 lux for dark phase experiments and 30 lux for light phase experiments.
Subjects used for the B6 versus BTBR comparison experiments were male mice born and raised at NIMH in Bethesda, MD, from original breeding pairs purchased from The Jackson Laboratory (Bar Harbor, ME). These strains were chosen for their divergent social behaviors, allowing the circadian questions to be addressed in mice with high social approach and with low social approach, and also to evaluate the replicability of previous findings (McFarlane et al., 2007
; Moy et al., 2007
). Light phase testing: B6, N = 11; BTBR, N = 12. Dark phase testing: B6, N = 12; BTBR, N = 12. Targeted disruption of the Avpr1b gene was described previously (Wersinger et al., 2002
). Avpr1b null mutants (−∕−), heterozygotes (+/−), and wildtype littermates (+/+) were bred from the offspring of non-sibling heterozygous. Subjects were male littermates generated by crossing non-sibling heterozygotes. Subjects of the current experiments were on an approximately equal mix of C57BL∕6J and 129X1∕SvJ background strains (Wersinger et al., 2004
).
To evaluate replicability of findings in mutant mice, four different investigators from our laboratory tested Avpr1b mutant mice in the automated three-chambered social approach task. Each investigator bred and tested his∕her own cohort(s) in the same vivarium and test rooms. The series of experiments was conducted during different seasons over the course of 1 year. Investigator T. C. performed light phase testing only (+/+, N = 13; +/−, N = 16; −∕−, N = 15). Investigator M. L. S. conducted dark phase testing only (+/+, N = 10; +/−, N = 12; −∕−, N = 12). Investigator H. G. M. conducted dark phase testing only (+/+, N = 12; +/−, N = 12; −∕−, N = 8). Investigator M. Y. conducted light and dark phase experiments simultaneously (dark phase: +/+, N = 9; +/−, N = 11; −∕−, N = 9; light phase: +/+, N = 10; +/−, N = 13; −∕−, N = 9).
Each mouse from the B6 and BTBR strain was used only once, as an adult in the social approach test. Mice from the vasopressin receptor 1b mutant line that were used for the juvenile play test was subsequently used for the adult social approach test (M. Y.). Adult vasopressin receptor 1b mutant mice used by other investigators had been tested in other experiments including maternal separation induced ultrasonic vocalizations (M. L. S.) and open field (H. G. M.).
Behavioral assays
Social approach behaviors were tested in an automated three-chambered apparatus using methods similar to those previously described (Crawley et al., 2007
; McFarlane et al., 2007
; Moy et al., 2004
, 2007
; Nadler et al., 2004
). Briefly, the apparatus was a rectangular, three-chambered box made from clear polycarbonate. Retractable doorways within the two dividing walls allowed access to the side chambers. Quantification of number of entries and time spent in the chambers was automatically measured by photocells embedded in the doorways. The apparatus was cleaned with 70% ethanol and water between subjects.
Animals used as “strangers” were male C57BL∕6J (T. C., M. S. L., H. G. M.) or 129Sv∕ImJ and AJ mice (M. Y.), aged 8–14 weeks old, bred in the NIMH vivarium from breeding pairs originally obtained from The Jackson Laboratory. Stranger mice were habituated to the apparatus and to the wire cup enclosure before the start of experiments, for 10 minutes per day for three consecutive days. The subject mouse was allowed to acclimate to the apparatus for 20 minutes before the sociability test, 10 minutes in the central chamber with the doors closed, followed by 10 minutes in the entire empty arena with the doors open. The subject was then briefly confined to the center chamber while a novel object (inverted wire cup, Galaxy Cup, Kitchen Plus, http://www.kitchen-plus.com
) was introduced into one of the side chambers. A stranger mouse enclosed in an identical wire cup was placed in the other side chamber. An upright plastic drinking cup, held in place by a lead weight in the cup, was placed on the top of each inverted wire cup to prevent the subject from climbing onto the top of the wire cup. Side chamber location of the novel object and the stranger mouse alternated between the left and right chambers across subjects. Lack of innate side preference was confirmed in previous experiments and during the 10 minutes habituation to the entire arena in the present experiments. After both stimuli were positioned, the doors were simultaneously re-opened and the subject was allowed access to all three chambers for 10 minutes. Measures taken included time spent in each chamber, time spent sniffing each cup, and the number of entries. An observer uninformed of the genotypes scored time spent sniffing with a stopwatch.
For the B6 and BTBR circadian comparison experiments, similar numbers of subjects from each strain were alternately tested on any test day. For the Avpr1b mice experiments, subjects of all genotypes from a home cage were tested in randomized order. When light and dark phase experiments were conducted simultaneously in a parallel design, light and dark tests were randomly alternated across days, but not within a single test day, due to the time-consuming procedure of properly rearranging lighting.
Juvenile play was analyzed in the Noldus PhenoTyper arena (Noldus, Leesburg VA, USA) as previously described (McFarlane et al., 2007
; Terranova and Laviola, 2005
). The juvenile play test was carried out at postnatal day 21 ± 1. One day before the play test, and 1-hour after being singly housed in a clean cage, each subject was allowed to acclimate to the entire empty arena for 10 minutes. The arena was cleaned with 70% ethanol and water between subjects. On the day of the play test, subjects were housed individually in the experimental room for 1 hour prior to the play test. Two non-sibling males of the same genotype were placed in the testing arena and their interactions were recorded for 30 minutes. Behaviors were subsequently scored from digital videotapes using the Noldus Observer 5.0 system, by a highly trained scorer unaware of the group assignment. Behaviors analyzed included nose sniffing, push past∕crawl over and under, huddling together, and follow, using definitions similar to previously described (McFarlane et al., 2007
, Supplementary Information). All behaviors were analyzed for frequency of occurrence, with the exception of huddling, for which duration was analyzed.
Statistical analysis
For the automated social approach task, Repeated Measures Analysis of Variance (ANOVA) was used to compare time spent in the chamber in the sociability test. Since times spent in each of the three chambers were not independent, the test condition factor compared time spent only in the right versus left chambers. Center chamber times are shown in the graphs for illustrative purposes. Time spent sniffing the novel object versus the stranger and entries to side chambers were similarly analyzed. Juvenile play parameters were analyzed using Multivariate Analysis of Variance (MANOVA). Newman–Keuls test was used for post-hoc pairwise comparisons following a significant overall F-value.
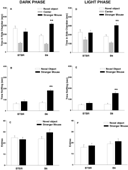
Figure 1
Panels A–C illustrate social approach behaviors of adult male B6 and BTBR mice housed on a reverse light cycle and tested in the dark phase. B6 spent significantly more time in the side chamber containing the stranger than in the side chamber containing the novel object (Figure 1
A, F1,10 = 28.17, p < 0.001). BTBR failed to spend more time in the side chamber containing the stranger (Figure 1
A, F1,11 = 0.37, NS). B6 spent more time sniffing the stranger than the novel object (Figure 1
B, F1,10 = 74.65, p < 0.0001). BTBR failed to spend more time sniffing the stranger (Figure 1
B, F1,11 = 4.44, p = 0.06). B6 and BTBR mice made similar numbers of entries between compartments (Figure 1
C, p = 0.388), indicating normal exploratory locomotion. Figure 1
Panels D–F illustrate social approach behaviors of adult male B6 and BTBR mice housed on a conventional light cycle and tested in the light phase. B6 spent significantly more time in the side chamber containing the stranger than in the side chamber containing the novel object (Figure 1
D, F1,11 = 9.11, p < 0.01). BTBR failed to spend more time in the side chamber containing the stranger (Figure 1
E, F1,11 = 0.20, NS). B6 spent more time sniffing the stranger than the novel object (Figure 1
E, F1,11 = 31.64, p < 0.001), BTBR failed to spend more time sniffing the stranger (Figure 1
E, F1,11 = 2.89, p = 0.12). B6 and BTBR mice made similar numbers of entries between compartments (Figure 1
F, p = 0.225), indicating normal exploratory locomotion. ANOVA analysis indicated that, overall, mice tested in the dark phase made more total entries than mice tested in the light phase (F1,90 = 21.24, p < 0.001). Circadian phase did not influence overall chamber time (F1,90 = 0.64, NS) or overall sniff time (F1,90 = 3.56, NS).
Figure 1. B6 mice displayed high levels of sociability while BTBR mice displayed low levels of sociability, when tested in either circadian phase. Left panels: dark phase testing of mice raised on a reverse light cycle; Right panels: light phase testing of mice raised on a conventional light cycle. (A) and (D) B6 spent significantly more time in the side chamber containing a stranger mouse than in the side chamber containing a novel object. BTBR spent approximately equal amounts of time in the side chambers containing a novel object and a stranger mouse; (B) and (E) B6 spent significantly more time sniffing the stranger than the novel object. BTBR did not; (C) and (F) number of entries into the side chambers was not significantly different between B6 and BTBR, indicating comparable levels of locomotor activity and exploratory tendencies. C57BL∕6J (B6), N = 11 (dark), N = 12 (light); BTBR T+tf∕J (BTBR), N = 12 (dark), N = 12 (light). **p < 0.01 for comparisons of the stranger mouse and novel object.
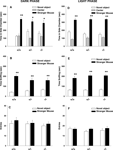
Figure 2
illustrates normal social approach to a stranger mouse in two cohorts of Avpr1b mice that were bred at the same time, one on a reverse light cycle and tested in the dark, and the other on a conventional light cycle and tested in the light. All three genotypes tested in the dark phase spent significantly more time in the chamber containing the stranger than in the chamber containing the novel object: (Figure 2
A, +∕+, F1,8 = 55.64, p < 0.0001; +∕−, F1,10 = 12.66, p < 0.05; −∕− F1,8 = 6.82, p < 0.05), spent more time sniffing the stranger than sniffing the novel object: (Figure 2
B, +∕+, F1,8 = 100.12, p < 0.0001; +∕−, F1,10 = 29.25, p < 0.001; F1,8 = 9.69, p < 0.01), and showed similar numbers of entries between compartments (Figure 2
C, p = 0.29). Social approach behaviors of all three genotypes of Avpr1b mice tested in the light phase showed significantly more time in the chamber containing the stranger than in the chamber containing the novel object: (Figure 2
D, +∕+, F1,9 = 14.48, p < 0.01; +∕− F1,12 = 30.30, p < 0.0001; −∕−, F1,8 = 50.05, p < 0.0001). All three genotypes spent more time sniffing the stranger than sniffing the novel object: (Figure 2
E, +∕ + , F1,9 = 25.85, p < 0.001; +∕− F1,12 = 38.77, p < 0.0001; F1,8 = 95.27, p < 0.0001), and showed similar numbers of entries between compartments (Figure 2
F, p = 0.83).
Figure 2. Vasopressin receptor subtype 1B (Avpr1b) null mutant (−∕−), heterozygous (+∕−), and wildtype littermate controls (+∕+) displayed normal sociability. Left panels: dark phase testing of mice raised on a reverse light cycle; right panels: light phase testing of mice raised on a conventional light cycle. (A) and (D) all three genotypes spent significantly more time in the chamber containing a stranger mouse than in the chamber containing a novel object; (B) and (E) all three genotypes spent significantly more time sniffing a stranger mouse than a novel object; (C) and (F) number of entries into the side chambers was not significantly different across genotypes, indicating similar locomotor activity and exploratory tendencies. +∕+ N = 9 (dark), N = 10 (light); +∕− N = 11 (dark), N = 13 (light); −∕− N = 9 (dark), N = 9 (light), *p < 0.05, **p < 0.01, within-group comparison between the stranger mouse and the novel object.
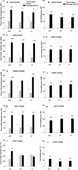
Figure 3
displays sociability data obtained from five separate experiments conducted with independent cohorts of Avpr1b mice. Dark phase testing is shown in panels A–F; light phase testing is shown in panels G–J. Panels A and B, taken from the experiments shown in Figures 2
A and 2
B, and panels G and H, taken from Figures 2
D and 2
E, are re-illustrated for comparison purposes. All three dark phase experiments and one light phase experiment revealed that all three genotypes spent more time in the chamber containing the stranger mouse than in the chamber containing the novel object. All three dark phase experiments and both light phase experiments showed that all three genotypes spent more time sniffing the stranger mouse than the novel object. Repeated measure ANOVA results of the five replications were: (A) Cohort 1, dark phase time in chamber, +∕ +, F1,8 = 55.64, p < 0.0001; +∕−, F1,10 = 12.66, p < 0.05; −∕− F1,8 = 6.82, p < 0.05; (B) Cohort 1, dark phase time spent sniffing, +∕ + , F1,8 = 100.12, p < 0.0001; +∕− F1,10 = 29.25, p < 0.001; F1,8 = 9.69, p < 0.01; (C) Cohort 2, dark phase time in chamber +∕+, F1,9 = 20.10, p < 0.001; +∕− F1,11 = 24.61, p < 0.001; −∕−, F1,11 = 24.89, p < 0.001; (D) Cohort 2, dark phase time spent sniffing, +∕+, F1,9 = 29.92, p < 0.001; +∕− F1,11 = 23.34, p < 0.001; −∕−, F1,11 = 74.69, p < 0.001; (E) Cohort 3, dark phase time in chamber, +∕+, F1,11 = 5.91, p < 0.05 +∕− F1,11 = 6.35, p < 0.05; −∕−, F1,7 = 23.93, p < 0.01; (F) Cohort 3, dark phase time spent sniffing, +∕+, F1,11 = 17.34, p < 0.01; +∕− F1,11 = 13.30, p < 0.01; −∕−, F1,7 = 45.74, p < 0.001. One light phase experiment demonstrated all three genotypes spent more time in the chamber containing the stranger mouse than the one containing the novel object: (G) Cohort 4, light phase time in chamber, +∕+, F1,9 = 14.48, p < 0.01; +∕− F1,12 = 30.30, p < 0.0001; −∕−, F1,8 = 50.05, p < 0.0001. (H) Cohort 4, light phase time spent sniffing, +∕+, F1,9 = 25.85, p < 0.001; +∕− F1,12 = 38.77, p < 0.0001; F1,8 = 95.27, p < 0.0001. (I) Cohort 5, light phase time in chamber, +∕+, F1,12 = 15.56, p < 0.01; +∕− F1,15 = 0.72, NS; −∕−, F1,14 = 0.001, NS. (J) Cohort 5, light phase time spent sniffing, +∕+, F1,12 = 12.58, p < 0.01; +∕− F1,15 = 21.91, p < 0.001; −∕−, F1,14 = 11.08, p < 0.01). All five replications found all three genotypes made similar numbers of entries to the side chambers (data not shown), indicating normal locomotor activity and exploratory tendencies.
Figure 3. Normal sociability of vasopressin receptor subtype 1B (Avpr1b) null mutants (−∕−), heterozygotes (+∕−), and wildtype littermate controls (+∕+) was seen in four out of five experiments, conducted by different investigators using independent cohorts of mice across a 1 year time span. (A–F) dark phase testing of mice raised on a reverse light cycle; (G–J) light phase testing of mice raised on a conventional light cycle; (A, C, E, G) all three genotypes spent significantly more time in the chamber containing a stranger mouse than in the chamber containing a novel object; (I) in one cohort, the wildtype group spent more time in the chamber containing a stranger mouse than in the chamber containing a novel object, while the heterozygous and null mutant mice failed to spend more time in the side chamber containing the stranger; (B, D, F, H, J) all three genotypes spent significantly more time sniffing the stranger mouse than the novel object. NS for each cohort were as listed in the text. *p < 0.05; **p < 0.01, within-group comparison between the stranger mouse side and the novel object side.
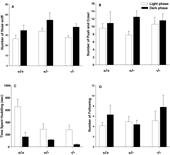
Figure 4
illustrates juvenile play behaviors in Avpr1b mice housed on a reverse light cycle and tested in the dark versus housed on a standard light cycle and tested in the light. Juvenile male play data were compared with MANOVA for phase, genotype, and phase × genotype interaction. No significant main effect of genotype was detected for nose sniff, F1,46 = 2.39, NS. Testing phase had a significant effect on nose sniff. F1,46 = 7.95, p < 0.01, with animals tested in the dark phase tending to display more nose sniffs than mice tested in the light phase. However, the interaction between testing phase and genotype was not significant. No significant effect of genotype or testing phase was detected for the number of push∕crawl (Genotype, F2,46 = 0.19, NS; Phase, F1,46 = 2.69, NS) or follow (Genotype, F2,46 = 0.77, NS; Phase, F1,46 = 1.40, NS). The main effect of testing phase was significant for total duration of huddling, F1,46 = 20.84, p < 0.0001. The main effect of genotype was significant, F1,46 = 5.48, p < 0.01. Phase × genotype interaction was not significant, F2,46 = 2.21, NS. Post-hoc Newman–Keuls test showed that Avpr1b +∕− and −∕− groups spent less time in physical contact, compared to +∕+ groups.
Figure 4. Juvenile male vasopressin receptor subtype 1b (Avpr1b) null mutant (−∕−), heterozygous (+∕−), and wildtype littermate controls (+∕+) exhibited similar levels of active push∕crawl (play soliciting behaviors) and nose sniff (social investigative behaviors) at PND 21 ± 1. Significant genotype differences were found for huddling, with heterozygotes null mutant pups spending less time huddling compared to wildtype pups. (A) nose sniff; (B) push and crawl; (C) huddling together; (D) following. +∕+, N = 8 (dark), N = 10 (light); +/−, N = 8 (dark), N = 10 (light); −∕−, N = 8 (dark), N = 8 (light). *p < 0.05 different from +∕+.
Mouse models of neuropsychiatric diseases offer translational tools for investigating genetic hypotheses. Mice are social animals with rich behavior repertoires, suitable for modeling human disorders characterized by social behavior deficits (Crawley, 2004
, 2007
; Panksepp and Lahvis, 2006
; Terranova et al., 1993
). Methods for analyzing social behaviors in mice require an understanding of test conditions that influence the results obtained. Since mice are more active at night (McLennan and Taylor-Jeffs, 2004
; Refinetti, 2004
; Whishaw et al., 1999
), the influence of circadian phase on social interactions is a critical parameter to understand when developing robust, replicable, high throughput assays of mouse social behaviors. In the present experiments, we evaluated social behaviors of three lines of mice, each with a cohort raised and tested in both the light and dark phases of the circadian cycle. Our primary finding is that testing phase does not have a strong impact on levels of social approach and interaction in two inbred strains of mice and in three genotypes of a knockout line of mice.
Both dark and light phase testing revealed marked differences in sociability between adult B6 and BTBR mice. B6 displayed clear tendencies to spend more time with a stranger mouse than with a novel object. BTBR mice failed to show such tendencies. The present findings, within a systematic comparison conducted simultaneously in the same laboratory, are consistent with previous findings of high sociability in B6 and low sociability in BTBR that were conducted in different laboratories with slightly different methods (McFarlane et al., 2007
; Moy et al., 2007
). The two strains exhibited similar levels of locomotor activities and exploratory tendencies in the social apparatus, confirming previous reports of normal procedural abilities relevant to social interaction in both strains (McFarlane et al., 2007
; Moy et al., 2007
). Of note, B6 tested in both phases spent similar amounts of time actively sniffing the stranger, indicating that animals tested in the light phase are fully alert and direct as much attention to a social stimulus as those tested in the dark phase. These findings demonstrated that testing phase and lighting levels do not have significant influences on the expression of sociability in the automated three-chambered social approach task, at least in the two inbred strains tested. Mice tested in the dark phase made more entries to the side chambers than those tested in the light phase, indicating that general exploratory activity is influenced by circadian phase. Nevertheless, neither the absolute levels of social approach behaviors nor strain differences in sociability was affected by variations in exploratory activities. Replicability of BTBR and B6 phenotypes on the social approach task, under different circadian conditions, in different laboratories, and across different cohorts of mice, thus appears to be robust.
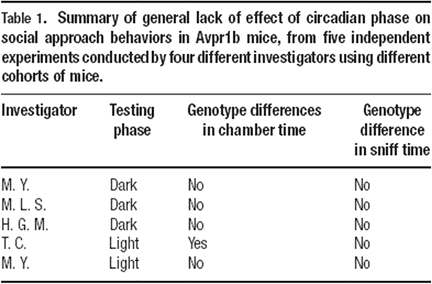
Sociability tests in Avpr1b null mutants (−∕−), heterozygotes (+∕−), and wildtype littermates (+∕+) tested in opposite circadian phases yielded similar results. One light phase and three dark phase experiments found that all three genotypes spent more time in the side chamber containing the stranger mouse than in the side chamber containing the novel object, and more time sniffing the stranger mouse than sniffing the novel object. One experiment, carried out in the light phase, revealed that heterozygotes and null mutants failed to spend more time in the chamber containing the stranger mouse than in the chamber containing the novel object. In this experiment, mice of all three genotypes did spend more time sniffing the stranger than the novel object. All five experiments yielded similar number of entries across genotypes, indicating normal exploratory locomotion. Therefore, as summarized in Table 1
, similar results were obtained in five out of five independent cohorts of Avpr1b mice, tested by different investigators at different times of the year, on two parameters in our social approach task, time spent sniffing the stranger mouse versus the novel object and entries into the side chambers. Four out of five independent cohorts yielded similar results on one parameter, time spent in the chamber containing the stranger versus the novel object. In addition, the absolute values for time spent in the side chambers and time spent sniffing were quite similar across experiments. These five datasets represent a reasonably high level of replicability within a line of mice tested on the social approach task.
Three representative behaviors were chosen to characterize active social interactions in male juvenile Avpr1b mice tested in opposite circadian phases. The best recognized play soliciting behaviors are pushing past, pushing under, and crawling over the other mouse (Terranova et al., 1993
, 1998
), which are combined in a single category labeled “push∕crawl” in the present study. Nose-to-nose sniff, an investigative behavior potentially analogous to eye contact in humans, has been postulated as a useful behavioral index in an animal model of autism (McFarlane et al., 2007
). Following, in which one mouse follows the other for a short amount of time, is commonly considered a social investigative behavior (File and Seth, 2003
; Terranova et al., 1998
). Our findings that genotype and testing phase had no significant effect on these juvenile Avpr1b behaviors are consistent with our social approach results obtained from the five cohorts of adult Avpr1b mutant mice. Huddling is a measure of inactivity that reflects two pups in physical contact, usually sitting quietly in a corner of the arena. We observed that huddling tends to increase toward the end of the 30-minute test session, probably reflecting that sufficient time has elapsed for pups to familiarize with each other, and for the competing behavior of exploring the novel environment to decrease, thus producing more sitting quietly in the corner. Our finding that huddling was higher in the light phase than in the dark phase is consistent with the tendency of mice to rest more in the light phase (Refinetti, 2004
). Note that the adult sociability test was only 10 minutes, whereas the juvenile play test was 30 minutes. Huddling typically did not occur in the first half of the 30 minutes, when arena exploration and active social interactions dominate. A genotype difference was detected, with Avpr1b +∕− and Avpr1b −∕− mice spending less time huddling compared to wildtype controls. Since huddling is the only behavior for which the genotype effect was significant, it is premature to interpret juvenile sociability with reference to the function of the Avpr1b gene. The present overall negative finding of the lack of effect of Avpr1b mutation on social interactions in mice is in contrast to a previous study which demonstrated that the same line of mice null for Avpr1b failed to spend more time sniffing soiled mice bedding as compared to clean bedding (Wersinger et al., 2004
). The discrepancy might be understood in light of the fact that live animals, which could be more potent social stimuli than soiled bedding, were used in the present study.
Mouse models are used extensively to study genetic mechanisms of behaviors. Issues of replicability of behavioral findings have been raised, even in extremely rigorously controlled studies (Crabbe et al., 1999
; Wahlsten et al., 2003
). It has been recommended that mouse behavior assays be standardized across laboratories to improve inter-laboratory replicability (Cryan and Mombereau, 2004
). Another view, perhaps more realistic, is that research environments can never be made similar enough to guarantee identical results (Wahlsten et al., 2003
). In our laboratory, investigators are trained to follow exactly the same experimental protocols for conducting the automated three-chambered social approach task. Great attention is given to procedural and environmental details to minimize inter-experimenter variability. The present results on social approach in five cohorts of Avpr1b mice show that the large majority of findings replicated very well, but even with such rigorous controls, identical results were not obtained for every measure in every cohort. The level of replicability for these social behavior parameters appears to be comparable to the level of replicability seen in other areas of biological research. Our findings support the recommendation that when a significant main effect of genotype is discovered, it is necessary to replicate the initial finding, as with other biological assays (Crabbe et al., 1999
; Crawley, 2007
).
The present finding that sociability was equally high when mice were tested in the light phase versus in the dark phase of the circadian cycle is interesting and unexpected. One possible explanation is that environmental factors in the vivarium and test facility may act as circadian entrainers. Field studies have shown that many species are not strictly nocturnal or diurnal, being able to adjust their behaviors according to environmental pressures (Mrosovsky, 2003
). Standard vivarium operations typically require routine cleaning, feeding, cage changes, and inspection of mice to be conducted during the light phase. Consequently, mice living under conventional light∕dark cycles, that is, the standard condition in most commercial and research facilities, are unavoidably disturbed during their normal resting phase. It is conceivable that animals that are better at adapting to such daily disruption have enjoyed superior reproductive success than those unable to adapt well. As a result, commonly used strains of laboratory mice might have been selected as useful research resources because they had evolved to be less strictly nocturnal, being able to adjust physiologically and behaviorally to the demands of the vivarium environment. Further, the ethological importance of investigating a novel conspecific may override the tendency of mice to sleep during the light phase. Thus, social interaction assays may be among the least sensitive to circadian phase, at least for laboratory mice.
In conclusion, the present data provide strong evidence that mice display qualitatively and quantitatively similar levels of social behaviors when raised on a conventional lighting cycle and tested during the light phase, as when raised on a reverse light cycle and tested during their dark phase. High sociability in B6, low sociability in BTBR, and the lack of genotype differences in Avpr1b mice, were generally unaffected by testing phase. Based on these findings, we suggest that it is methodologically appropriate to test mouse social approach behaviors in either circadian phase.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Ms. Tabitha Morris, NIMH, for her assistance in conducting components of the experiments shown in Figure 4 and Mr. James Heath, NIMH, for genotyping the Avpr1b mice. This research work was supported by the National Institute of Mental Health Intramural Research Program, Z01-MH-02179 (JNC) and Z01-MH-002498-17 (WSY, HC).
Crawley, J. N., Chen, T., Puri, A., Washburn, R., Sullivan, T. L., Hill, J. M., Young, N. B., Nadler, J. J., Moy, S. S., Young, L. J., Caldwell, H. K., and Young, W. S. (2007). Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides 41, 145–163.
Jennings, M, Batchelor, G. R., Brain, P. F., Dick, A., Elliott, H., Francis, R. J., Hubrecht, R. C., Hurst, J. L., Morton, D. B., Peters, A. G., Raymond, R., Sales, G. D., Sherwin, C. M., and West, C. (1998). Refining rodent husbandry: the mouse. Report of the Rodent Refinement Working Party. Lab. Anim. 32, 233–259.