Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis
- 1 Laboratory of Nutrition and Integrative Neurobiology, Université de Bordeaux-IPB, Talence, France
- 2 Faculty for Chemistry and Biochemistry, Department of Molecular Neurobiochemistry, Ruhr-University, Bochum, Germany
- 3 Faculty of Biology and Biotechnology, Department of Developmental Neurobiology, Ruhr-University, Bochum, Germany
Neurogenesis occurs in two neurogenic zones in the adult brain: new neurons are born at the subventricular zone of the lateral ventricles and then migrate to the olfactory bulb, and at the subgranular zone to integrate the granular cell layer of the dentate gyrus. The hippocampus is involved in learning and memory and the generation of new hippocampal neurons has been suggested to be a new form of plasticity implicated in these processes. In the last decades, diverse intrinsic and epigenetic factors have been identified to influence adult neurogenesis but the underlying mechanisms remain unclear. In a recent study, Lafenetre et al. (2010) showed the beneficial influence of physical voluntary activity on adult neurogenesis and cognitive performance in a transgenic mouse, the synRas mouse via brain-derived neurotrophic factor. Here we review how hippocampal neurogenesis can be regulated by environmental factors and the possible role of the newly generated cells in learning and memory.
Introduction
Altman and Das (1965) proposed in 1965 the revolutionary idea that new neurons are born in the adult brain. For decades, the concept has been ignored until it was rediscovered in the early 1990s. It is now well accepted that new neurons are born throughout life, even in humans (Eriksson et al., 1998; Jin et al., 2004; D’Alessio et al., 2010) and in aged animals (Kuhn et al., 1996; Kempermann et al., 1998a). Adult neurogenesis has been clearly demonstrated and confirmed in two brain regions: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampal formation (Kempermann and Gage, 1999). Cells born in the adult SVZ migrate through the rostral migratory stream and become granule neurons and periglomerular neurons in the olfactory bulb. Cells born in the adult SGZ migrate into the granule cell layer of the DG and become dentate granule cells (DGC).
It is only in the last decade that hippocampal adult neurogenesis has become a hot topic. Many studies have been designed to characterize these newly generated neuronal cells (Kempermann et al., 2004; Zhao et al., 2006), to unravel the regulation of the different maturation stages (Zhao et al., 2008) and to unveil the potential meaning of this phenomenon (Zhao et al., 2008; Deng et al., 2010).
The newly generated cells undergo different maturation stages to become functional mature neurons. As a consequence of neurons being born in a continuous manner, the DG is composed of a heterogeneous population of dividing and non-dividing, immature and mature, neuronal and non-neuronal cells. It has thus been difficult to understand the progression of these developmental steps. Many studies have first examined the expression of diverse cell markers. Kempermann et al. (2004) have developed a model of six developmental milestones of hippocampal adult neurogenesis based on their basic morphology and the combinatorial expression of six fairly stage-specific markers. Type-1 cells are the putative stem cells. They are characterized by their radial process extending to the inner molecular layer where they ramify, and by the expression of both nestin and glial fibrillary acidic protein (GFAP). Nestin-positive cells that do not express GFAP nor doublecortin are classified as type-2a putative progenitor cells. Doublecortin is indeed expressed in type-2b, type-3 cells, both putative progenitor cells, and by immature granule cells. Type-2b cells display an irregularly shaped dense nucleus and the coexpression of nestin. Type-3 cells have a rounded nucleus and do not express nestin nor GFAP. Postmitotic immature neurons are further characterized by the emergence of dendrites and the coexpression of calretinin or NeuN. Finally, mature DGC have developed a dendritic arborization and are calbindin- and NeuN-positive. Recent studies have suggested that the electrophysiological and morphological properties of the newborn cells gradually emerge during neuronal maturation. These properties are needed by the young neurons for successful integration into the existing synaptic circuits (Zhao et al., 2006, 2008; Aimone et al., 2010).
The hippocampus is undisputedly one of the brain centers essential for learning and memory. The idea that new neurons are needed to build new memories has been quick to emerge. However, easily generated ideas are often difficult to prove. The concept implies hippocampal adult neurogenesis as being a new form of structural plasticity correlated to the processes of synaptic learning and memory formation. Should this be the case, animals with impaired neurogenesis should, compared to wildtype animals, show alterations in acquisition, retention, recall or extinction of some kinds of information like spatial or contextual information.
Lafenetre et al. (2010) have used the synRas transgenic mouse model in order to better understand the role of adult neurogenesis in learning and memory. The mouse has a synapsin1 promoter-driven overexpression of the constitutively activated G-protein p21Ras in neurons. Intriguingly, this mouse has significantly depressed rates of hippocampal adult neurogenesis, and this is associated with impaired performance in an object recognition task (Lafenetre et al., 2010). Both, impaired neurogenesis and impaired object recognition could however be rescued by exposing synRas mice to free access to a running wheel (Lafenetre et al., 2010; Table 1).
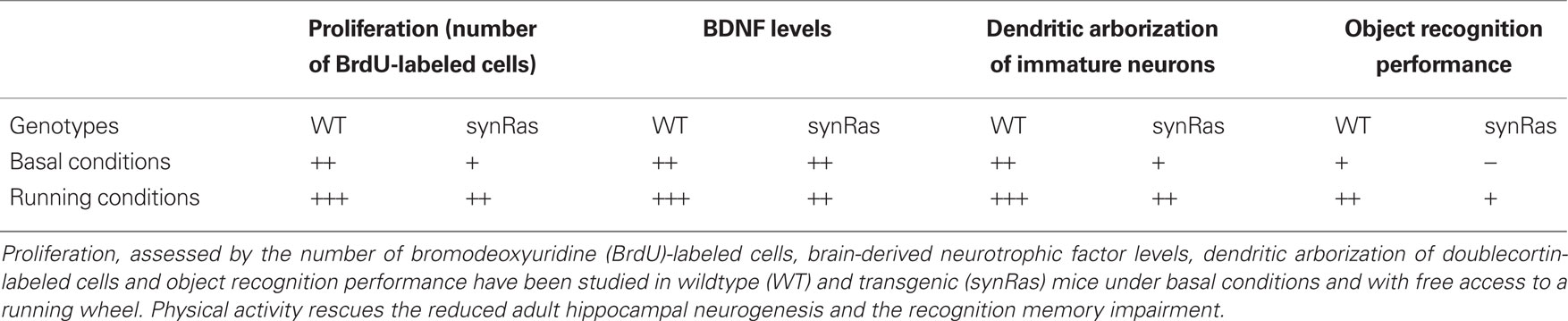
Table 1. Main results at a glance (Lafenetre et al., 2010).
Here we review the study by Lafenetre et al. (2010), starting by presenting the regulation of adult neurogenesis by physical activity as an epigenetic factor and the possible implication of neurotrophins as molecular mediators. We will finally discuss the relationships between adult neurogenesis and learning and memory.
Epigenetic Regulation of Adult Neurogenesis
Adult neurogenesis has been shown to be a dynamic process that can be regulated both positively and negatively by many factors, including epigenetic factors. Indeed, in the last decades, five of them have clearly been identified and studied. Whereas stress and aging downregulate adult neurogenesis, physical activity, environmental enrichment, and learning and memory have beneficial effects (Figure 1).
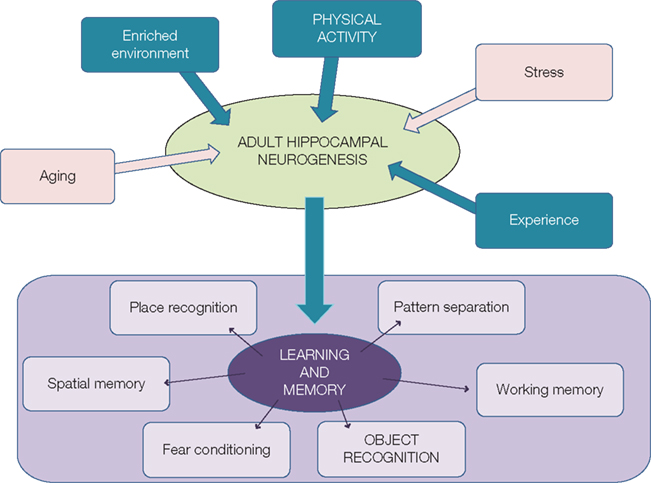
Figure 1. Regulation of adult neurogenesis can modulate learning and memory. Whereas aging and stress downregulate adult neurogenesis (pink arrows), experience, enriched environment and physical activity (blue arrows) stimulate the generation of new neurons. This could thus lead to better performance in learning and memory tasks.
Physical activity is the environmental factor that has been selected by Lafenetre et al. (2010) to rescue the reduced adult neurogenesis of the synRas mice due to reduced proliferation. Voluntary physical activity is indeed the most potent enhancer of adult proliferation (van Praag et al., 1999a; Olson et al., 2006; Fabel and Kempermann, 2008). The increase in neurogenesis is however region-specific and occurs only in the hippocampus and does not stimulate the olfactory bulb neurogenesis (Brown et al., 2003).
In order to study the influence of physical activity in rodents, a running wheel is usually introduced in their home cage. However, the modalities could vary from one experiment to another and could induce differential effects. First, the access to the wheel could be free or restricted to some hours per day or night (Holmes et al., 2004). Second, running is limited to physical activity when rodents are housed individually but could also be considered as a social activity when the animals are housed per group. Indeed, when rodents are isolated, the effects may be delayed or prevented (Stranahan et al., 2006; Leasure and Decker, 2009) but this is still under debate (Kannangara et al., 2009, 2010). Thirdly, grouped animals could use the running wheel according to two modes: some are very “active” and seem to become addicted to running whereas other individuals are “passive users.” In the study by Lafenetre et al. (2010), grouped mice had free access to the running wheel: this paradigm gave the greatest opportunity to enhance adult neurogenesis.
Lafenetre et al. (2010) have thus confirmed the positive regulation of adult neurogenesis by physical activity that had been described earlier in wild type rodents (van Praag et al., 1999a,b; Fabel et al., 2003; Farmer et al., 2004). This paradigm is also efficient in stimulating the neurogenesis of synRas mice characterized by a reduced basal adult neurogenesis in the hippocampus (Figure 2; Lafenetre et al., 2010; Manns et al., 2010) to an extent comparable to that in wildtype animals. The ways by which physical activity promotes proliferation must thus differ from the regulation of proliferation in basal conditions (Lafenetre et al., 2010). It has earlier been shown that neurogenesis could be partially rescued in aged animals by voluntary physical activity. This also suggests the regulatory mechanisms could be modified during aging but that the cells could still respond to external stimuli (van Praag et al., 2005; Kronenberg et al., 2006).
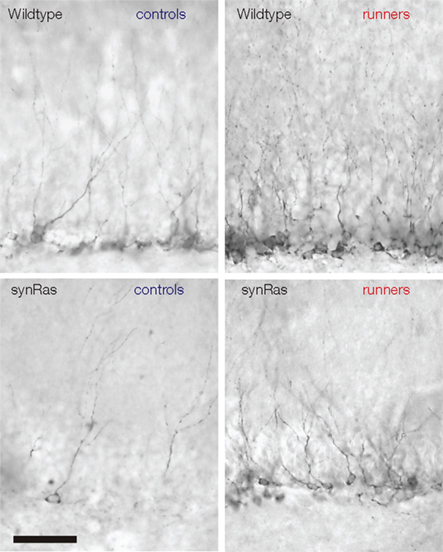
Figure 2. Physical activity could stimulate adult neurogenesis in the synRas mice. Doublecortin-labeled cells in the dentate gyrus of wildtype controls (upper left), wildtype runners (upper right), synRas controls (bottom left), and synRas runners (bottom right).
Wheel running has also been shown to improve hippocampal-dependent spatial learning in rodents in the Morris water maze (Fordyce and Farrar, 1991; van Praag et al., 1999a; Vaynman et al., 2004) and in the radial maze (Anderson et al., 2000). Other tasks like contextual fear conditioning have also been used to show the running-induced improvement in cognitive performance (Baruch et al., 2004).
The beneficial effect of physical activity could be mediated by increased synaptic plasticity (van Praag et al., 1999b; Farmer et al., 2004), neurotransmission and growth factor expression (Cotman and Berchtold, 2002) that are observed both in running mice and rats. In their study, Lafenetre et al., (2010) have focused on the involvement of brain-derived neurotrophic factor (BDNF) as one of the potential mediators of these effects.
Neurotrophins and Adult Neurogenesis
Brain-derived neurotrophic factor is a neurotrophin that is highly expressed in the hippocampus and has been implicated in synaptic plasticity and neuronal development (Binder and Scharfman, 2004). BDNF is known for its survival-promoting effects on new neuroblasts through the TrkB receptor (Bath et al., 2008). Environmental factors like environmental enrichment and physical activity induce an increase in BDNF expression level even after a short exposure to a running wheel (Cotman and Berchtold, 2002). Moreover, running wheel activity increases the levels of the phosphorylated form of the BDNF receptor TrkB (Vaynman et al., 2003).
The functional role of BDNF in the regulation of the hippocampal adult neurogenesis is quite controversial. Lafenetre et al. (2010) showed a running-induced increase in BDNF protein level that was associated with increased level of bromodeoxyuridine (BrdU)-labeled cells and doublecortin-labeled cells in both wildtype and synRas mice. BDNF acts through the TrkB receptor which is present on doublecortin-expressing cells (Donovan et al., 2008; Lafenetre et al., 2010). The authors thus suggested that BDNF could not only promote the proliferation of doublecortin-expressing neuronal precursor cells but also stimulate the dendritic arborization of the immature neurons and facilitate their survival (Figure 3).
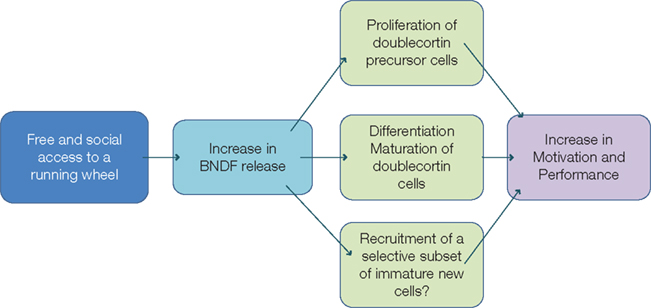
Figure 3. Working hypothesis. Free and social access to the running wheel would induce an increase in BDNF release that could act on (1) the proliferation of doublecortin-immunoreactive precursor cells, (2) on the dendritic arborization of doublecortin-immunoreactive immature neurons, stimulating their maturation and their differentiation, (3) on the selective recruitment of immature cells. These actions would thus lead to increased motivation and better cognitive performance.
The infusion of BDNF for 2 weeks directly into the hippocampus increases neurogenesis of granule cells (Scharfman et al., 2005). However, classical genetic studies manipulating directly the expression of BDNF do not lead to such clear results. Indeed, enhanced and reduced cell proliferation in heterogeneous BDNF knockout (BDNF+/−) mice have been reported (Lee et al., 2002; Sairanen et al., 2005). The survival rate of the neurons must however be dependent on BDNF expression as impaired levels of cell survival were observed in both studies (Lee et al., 2002; Sairanen et al., 2005). Moreover, enriched environment does not enhance the survival of newborn cells in BDNF+/− heterogenous knockout mice (Rossi et al., 2006).
In accordance with the role of BDNF in promoting the dendritic differentiation of mature neurons (McAllister et al., 1996; Wirth et al., 2003), the dendritic arborization of the DGC is more developed in BDNF-overexpressing transgenic mice (Tolwani et al., 2002). In a recent study, the role of BDNF in different stages of adult neurogenesis has been assessed in conditional knockout mice that lack the expression of BDNF in mature neurons of the adult hippocampus, resulting in 50% of the BDNF levels. BDNF has been suggested to play a critical role in the regulation of the survival and of the dendritic development of neuronal precursor cells but seems less important for exercise-induced proliferation of the cells (Choi et al., 2009). The conditional deletion of the TrkB receptor in progenitor cells leads to impaired basic organization of synaptic connections and compromises the survival and the integration of the newborn neurons (Bergami et al., 2008). Thus, whereas the role of BDNF in the proliferation of neuronal progenitor cells is still unclear, the survival and the integration of these newborn neurons rely on the good functioning of BDNF/TrkB signaling.
Due to the cellular heterogeneity of the DG, the understanding of the role of BDNF and other growth and neurotrophic factors, like VEGF, IGF1, Erythropoietin, remains difficult. Presumably, they have to be tightly regulated and can act differentially on different developmental stages of the various cellular subsets.
Adult Neurogenesis and Learning and Memory
What is adult neurogenesis good for? The functionality of the newly generated neurons has remained incompletely understood. With regard to the well-characterized role of the hippocampus in learning and memory, hippocampal adult neurogenesis has been proposed to be a new form of plasticity that underlies these processes.
Three technical approaches have been used to study the involvement of the newly generated neurons in learning and memory processes: the neural progenitor cells are ablated by pharmacological treatment, by irradiation or by genetic tools. Neural progenitor cells are indeed more vulnerable than mature granule cells. Injections of the DNA methylating agents, methylazoxymethanol (MAM), or more recently of Temozolomide (TMZ), significantly reduce the rate of adult neurogenesis. X-ray irradiation has also been a very powerful, even stronger, tool. However, these techniques may induce side-effects that could cause other detrimental effects on brain physiology and function (Bruel-Jungerman et al., 2007). With the generation of new genetic tools, it has been possible to specifically target the neural progenitor cells in the hippocampus and better assess the role of the new neurons in learning and memory processes.
Besides, the use of other transgenic mice has helped in the better characterization of some molecules that could be involved in the regulation of adult neurogenesis. As in Lafenetre et al., (2010), many studies have used environmental stimuli that could affect both, the animals’ behavior and adult neurogenesis (Figure 3).
Deng et al. (2010) have recently reviewed the different behavioral tasks that have been performed with animals subjected to an ablation of progenitor cells. Controversial results have been obtained using similar experimental paradigms. For instance, the involvement of adult neurogenesis in the acquisition of the Morris water maze test, the most commonly used paradigm to test hippocampal functions, has been reported by Dupret et al. (2008), Farioli-Vecchioli et al. (2008), Garthe et al. (2009), and Zhang et al. (2008). By contrast, other studies have reported that adult neurogenesis is not required for the acquisition of the Morris water maze test (Shors et al., 2002; Madsen et al., 2003; Raber et al., 2004; Rola et al., 2004; Snyder et al., 2005; Deng et al., 2009; Jessberger et al., 2009). Similarly, is adult neurogenesis necessary for long-term retention of the location of the hidden platform? When progenitor cells are ablated by irradiation (rat; Snyder et al., 2005), viral disruption of the Wnt signaling (rat; Jessberger et al., 2009) and genetic disruption (mouse; Dupret et al., 2008; Farioli-Vecchioli et al., 2008; Zhang et al., 2008), performances are impaired. However, the retention is only affected 1 week after the viral injection in the genetic model of Deng et al., (2009) and only the retention of the reversal is affected by TMZ treatment (Garthe et al., 2009). Other studies have not found any positive correlation between reduced adult neurogenesis and impaired cognitive performance in the Morris water maze (Shors et al., 2002; Meshi et al., 2006; Saxe et al., 2006) and it has even been revealed that inhibiting adult neurogenesis could lead to better learning and memory performances (Kerr et al., 2010).
Similar contradictory results have been obtained in the contextual fear conditioning paradigm which is also a key test to assess the role of the hippocampus. Some studies have found deficits in freezing behavior (Saxe et al., 2006; Winocur et al., 2006; Imayoshi et al., 2008; Warner-Schmidt et al., 2008; Hernandez-Rabaza et al., 2009; Snyder et al., 2009) but no effect on freezing were reporting by others (Dupret et al., 2008; Zhang et al., 2008; Snyder et al., 2009). While specific effects on the formation but not the extinction of contextual fear memory have been found in strongly irradiated mice, mice that have received MAM injections (Ko et al., 2009) or mice that have been irradiated to a lesser extent (Kitamura et al., 2009; Ko et al., 2009) are not impaired. By contrast, in Nestin-tk mice with ablation of actively dividing progenitor cells by ganciclovir, impairment in freezing or fear conditioning is not observed, yet they are not able to extinguish their fear response to the context as efficiently as the wildtype mice (Deng et al., 2009).
Lafenetre et al. (2010) have proposed that the newly generated neurons are important for object recognition. The synRas mouse has a reduced adult neurogenesis and is impaired in exploring and discriminating a novel object compared to a familiar object. However, stimulating adult neurogenesis by physical activity rescues these deficits (Table 1). Similar deficits have been observed in rats that have been injected with a lentiviral virus targeting the Wnt signaling (Jessberger et al., 2009). However, irradiation does not lead to comparable deficits (Madsen et al., 2003; Rola et al., 2004; Clelland et al., 2009). Several other studies employing hippocampus-dependent or independent tasks have also led to inconclusive results (see Deng et al., 2010 for review).
Such discrepancies could reflect the importance of many factors. For instance, species and strain/substrain differences of brain endophenotype are crucial. Recent studies have shown how dramatic hippocampal activity varies between standard laboratory mouse lines including those used for genetic engineering. Electrical activity of neuronal networks is a driving force, e.g., for the production, release and action of BDNF, the structural differentiation of neurons and the ability to form meaningful circuits. In particular, mouse lines differ in the patterns of spontaneous and evoked gamma oscillations, which are considered to be central to cognitive performance (Jansen et al., 2009). Strains also differ in the rates of proliferation and/or survival of newly formed cells (Kempermann and Gage, 2002) and this correlates with deficits in learning tasks (Kempermann et al., 1998b). Even substrains of the frequently used C57 strain differ dramatically in many behavioral aspects (Siegmund et al., 2005; Matsuo et al., 2010). The different ablation protocols may also affect different populations of cells that would be more vulnerable according to their maturation stage (Deng et al., 2010). Equally important are the collateral side-effects, the parameters used to assess the different phenotypes, the cognitive demand of the test, age, sex, and presumably the life history of the individual animals. It is thus difficult to integrate the results of different studies with so many variables. It would thus be needed to standardize protocols to target stage-specific cells in specific conditions.
An often overlooked aspect is that most experimental paradigms are primarily designed to assess the role of the hippocampus with all its input, intrinsic, and output networks. Are the tests adequate for understanding the role of the newly generated granule cells in the DG? Of course, the DG is considered as the information gateway to the hippocampus and has been specifically shown to be involved in pattern separation (Gilbert et al., 2001; McHugh et al., 2007). Computational models based on this function of the DG have been proposed for explaining either the addition of the new neurons in the network or the replacement of old DGC by these new neurons (Aimone et al., 2009, 2010; Deng et al., 2010). New born cells would thus help avoiding interferences between new and already established memories and promoting the individuals’ behavioral adaptation. Studies directly assessing this role are thus needed to better understand the real involvement of adult neurogenesis in learning and memory processes. According to a recent study, the newly generated cells in the DG must have a functional implication in pattern separation only when stimuli are presented with little separation in space, but not when they are widely separated (Clelland et al., 2009). Similarly, voluntary running improves the performance of the subtle discrimination of the location of two adjacent identical stimuli, which is tightly correlated to adult neurogenesis (Creer et al., 2010). Adult new neurons may thus be important for pattern separation and for encoding fine spatial distinctions (Clelland et al., 2009; Creer et al., 2010).
Conclusion
Lafenetre et al., (2010) have been able to show that voluntary physical activity could rescue two main phenotypes, the reduced adult neurogenesis and the impaired performance in an object recognition test, of a genetically modified mouse via an increase of BDNF. These results thus support the idea that adult neurogenesis is a dynamic process that is under the influence of environmental changes and that this form of plasticity is regulated by neurotrophic factors like BDNF.
Epigenetic factors, physical activity, enriched environment, aging, stress, and learning induce changes in both, the rate of adult neurogenesis and the behavior of the individual. It thus appears straight forward to associate both phenomena. However, the causal relationships are still not clearly determined and the role of adult neurogenesis in learning and memory processes remain obscure due to the many discrepant results. More studies are needed to better determine the effects of the various extrinsic factors on the progenitors and the subsequent stages of differentiation of the newly generated neurons, and their ability to integrate into the adult DG. Furthermore, the functional involvement of the newly generated granule cells must be further studied in the restricted context of the direct role of the DG. Computational models will thus help in elaborating theories that could be tested with animal models.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the DFG Graduate Programme 736 and the GK Development and Plasticity of the Nervous System for their financial support.
Key Concepts
Generation of new neurons in the adult brain throughout life in a multi-step process including the proliferation of the neural progenitor cells, the fate determination, the differentiation and the migration, the morphogenesis and the maturation of the neuronal cells and the integration into the neuronal network.
Neurotrophins are proteins that are capable of signaling particular cells in an autocrine or paracrine manner to proliferate, survive, differentiate, or grow. There are four neurotrophins: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4). They can activate the Ras/MAPK signaling cascade by binding to specific tropomyosin-receptor-kinase receptors with preferential affinities: NGF to TrkA, BDNF and NT-4 to TrkB, NT-3 to TrkC. They can also bind to the p75 neurotrophin receptor.
Epigenetics, as opposed to genetics, studies the interplay between environment and genes, in particular the regulation of gene expression, which results in a given phenotype. In adult neurogenesis, intrinsic and extrinsic factors alter the pattern of DNA methylation within the dentate dyrus (Covic et al., 2010). In this review, we consider environmental factors and experience as extrinsic epigenetic factors.
Process allocated to the dentate gyrus that renders the output firing patterns in a network less similar then the input firing patterns, either with neurons firing at different rates or with a different set of neurons. This would help avoiding interferences between memories to be encoded by the CA regions of the hippocampus.
References
Aimone, J. B., Deng, W., and Gage, F. H. (2010). Adult neurogenesis: integrating theories and separating functions. Trends Cogn. Sci. 14, 325–337.
Aimone, J. B., Wiles, J., and Gage, F. H. (2009). Computational influence of adult neurogenesis on memory encoding. Neuron 61, 187–202.
Altman, J., and Das, G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335.
Anderson, B. J., Rapp, D. N., Baek, D. H., McCloskey, D. P., Coburn-Litvak, P. S., and Robinson, J. K. (2000). Exercise influences spatial learning in the radial arm maze. Physiol. Behav. 70, 425–429.
Baruch, D. E., Swain, R. A., and Helmstetter, F. J. (2004). Effects of exercise on Pavlovian fear conditioning. Behav. Neurosci. 118, 1123–1127.
Bath, K. G., Mandairon, N., Jing, D., Rajagopal, R., Kapoor, R., Chen, Z. Y., Khan, T., Proenca, C. C., Kraemer, R., Cleland, T. A., Hempstead, B. L., Chao, M. V., and Lee, F. S. (2008). Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J. Neurosci. 28, 2383–2393.
Bergami, M., Rimondini, R., Santi, S., Blum, R., Gotz, M., and Canossa, M. (2008). Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. U.S.A. 105, 15570–15575.
Binder, D. K., and Scharfman, H. E. (2004). Brain-derived neurotrophic factor. Growth Factors 22, 123–131.
Brown, J., Cooper-Kuhn, C. M., Kempermann, G., van Praag, . H., Winkler, J., Gage, F. H., and Kuhn, H. G. (2003). Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–2046.
Bruel-Jungerman, E., Rampon, C., and Laroche, S. (2007). Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev. Neurosci. 18, 93–114.
Choi, S. H., Li, Y., Parada, L. F., and Sisodia, S. S. (2009). Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol. Neurodegener. 4, 52.
Clelland, C. D., Choi, M., Romberg, C., Clemenson, G. D. Jr., Fragniere, A., Tyers, P., Jessberger, S., Saksida, L. M., Barker, R. A., Gage, F. H., and Bussey, T. J. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213.
Cotman, C. W., and Berchtold, N. C. (2002). Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301.
Covic, M., Karaca, E., and Lie, D.C. (2010). Epigenetic regulation of neurogenesis in the adult hippocampus. Heredity, 105, 122–134.
Creer, D. J., Romberg, C., Saksida, L. M., Van Praag, H., and Bussey, T. J. (2010). Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. U.S.A. 107, 2367–2372.
D’Alessio, L., Konopka, H., Lopez, E. M., Seoane, E., Consalvo, D., Oddo, S., Kochen, S., and Lopez-Costa, J. J. (2010). Doublecortin (DCX) immunoreactivity in hippocampus of chronic refractory temporal lobe epilepsy patients with hippocampal sclerosis. Seizure 19, 567–572.
Deng, W., Aimone, J. B., and Gage, F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350.
Deng, W., Saxe, M. D., Gallina, I. S., and Gage, F. H. (2009). Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542.
Donovan, M. H., Yamaguchi, M., and Eisch, A. J. (2008). Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus 18, 435–439.
Dupret, D., Revest, J. M., Koehl, M., Ichas, F., De Groot, F., Costet, P., Abrous, D. N., and Piazza, P. V. (2008). Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 3, e1959. doi: 10.1371/journal. pone.0001959
Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., and Gage, F. H. (1998) Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317.
Fabel, K., Fabel, K., Tam, B., Kaufer, D., Baiker, A., Simmons, N., Kuo, C. J., and Palmer, T. D. (2003). VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812.
Fabel, K., and Kempermann, G. (2008). Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular. Med. 10, 59–66.
Farioli-Vecchioli, S., Saraulli, D., Costanzi, M., Pacioni, S., Cina, I., Aceti, M., Micheli, L., Bacci, A., Cestari, V., and Tirone, F. (2008). The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 6, e246. doi: 10.1371/journal.pbio.0060246
Farmer, J., Zhao, X., van Praag, H., Wodtke, K., Gage, F. H., and Christie, B. R. (2004). Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124, 71–79.
Fordyce, D. E., and Farrar, R. P. (1991). Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav. Brain Res. 46, 123–133.
Garthe, A., Behr, J., and Kempermann, G. (2009). Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE 4, e5464. doi: 10.1371/journal. pone.0005464
Gilbert, P. E., Kesner, R. P., and Lee, I. (2001). Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus 11, 626–636.
Hernandez-Rabaza, V., Llorens-Martin, M., Velazquez-Sanchez, C., Ferragud, A., Arcusa, A., Gumus, H. G., Gomez-Pinedo, U., Perez-Villalba, A., Rosello, J., Trejo, J. L., Barcia, J. A., and Canales, J. J. (2009). Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience 159, 59–68.
Holmes, M. M., Galea, L. A., Mistlberger, R. E., and Kempermann, G. (2004). Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J. Neurosci. Res. 76, 216–222.
Imayoshi, I., Sakamoto, M., Ohtsuka, T., Takao, K., Miyakawa, T., Yamaguchi, M., Mori, K., Ikeda, T., Itohara, S., and Kageyama, R. (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153–1161.
Jansen, R., Linkenkaer-Hansen, K., Heistek, T., Timmerman, J., Mansvelder, H. D., Brussaard, A. B., de Gunst, G. M., and van Ooyen, O. A. (2009). Inbred mouse strains differ in multiple hippocampal activity traits. Eur. J. Neurosci. 30, 1092–1100.
Jessberger, S., Clark, R. E., Broadbent, N. J., Clemenson, G. D. Jr., Consiglio, A., Lie, D. C., Squire, L. R., and Gage, F. H. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 16, 147–154.
Jin, K., Peel, A. L., Mao, X. O., Xie, L., Cottrell, B. A., Henshall, D. C., and Greenberg, D. A. (2004). Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 101, 343–347.
Kannangara, T. S., Lucero, M. J., Gil-Mohapel, J., Drapala, R. J., Simpson, J. M., Christie, B. R., and van Praag, H. (2010). Running reduces stress and enhances cell genesis in aged mice. Neurobiol. Aging. doi:10.1016/j. neurobiolaging.2009.12.025. [Epub ahead of print].
Kannangara, T. S., Webber, A., Gil-Mohapel, J., and Christie, B. R. (2009). Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice. Hippocampus 19, 889–897.
Kempermann, G., and Gage, F. H. (2002). Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res. Dev. Brain Res. 134, 1–12.
Kempermann, G., Jessberger, S., Steiner, B., and Kronenberg, G. (2004). Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447–452.
Kempermann, G., Kuhn, H. G., and Gage, F. H. (1998a). Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 18, 3206–3212.
Kempermann, G., Brandon, E. P., and Gage, F. H. (1998b). Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr. Biol. 8, 939–942.
Kerr, A. L., Steuer, E. L., Pochtarev, V., and Swain, R. A. (2010). Angiogenesis but not neurogenesis is critical for normal learning and memory acquisition. Neuroscience 171, 214–226.
Kitamura, T., Saitoh, Y., Takashima, N., Murayama, A., Niibori, Y., Ageta, H., Sekiguchi, M., Sugiyama, H., and Inokuchi, K. (2009). Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139, 814–827.
Ko, H. G., Jang, D. J., Son, J., Kwak, C., Choi, J. H., Ji, Y. H., Lee, Y. S., Son, H., and Kaang, B. K. (2009). Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol. Brain 2, 1.
Kronenberg, G., Bick-Sander, A., Bunk, E., Wolf, C., Ehninger, D., and Kempermann, G. (2006). Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging 27, 1505–1513.
Kuhn, H. G., Dickinson-Anson, H., and Gage, F. H. (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033.
Lafenetre, P., Leske, O., Ma-Hogemeier, Z., Haghikia, A., Bichler, Z., Wahle, P., and Heumann, R. (2010). Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Front. Behav. Neurosci. 3:34. doi: 10.3389/ neuro.08.034.2009
Leasure, J. L., and Decker, L. (2009). Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus 19, 907–912.
Lee, J., Seroogy, K. B., and Mattson, M. P. (2002). Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 80, 539–547.
Madsen, T. M., Kristjansen, P. E., Bolwig, T. G., and Wortwein, G. (2003). Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 119, 635–642.
Manns, M., Bichler, Z., Leske, O., and Heumann, R. (2010). Neuronal Ras activation inhibits adult hippocampal progenitor cell division and impairs spatial short-term memory. Genes Brain Behav. 9, 525–536.
Matsuo, N., Takao, K., Nakanishi, K., Yamasaki, N., Tanda, K., and Miyakawa, T. (2010). Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 4:29. doi: 10.3389/fnbeh.2010.00029
McAllister, A. K., Katz, L. C., and Lo, D. C. (1996). Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17, 1057–1064.
McHugh, T. J., Jones, M. W., Quinn, J. J., Balthasar, N., Coppari, R., Elmquist, J. K., Lowell, B. B., Fanselow, M. S., Wilson, M. A., and Tonegawa, S. (2007). Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99.
Meshi, D., Drew, M. R., Saxe, M., Ansorge, M. S., David, D., Santarelli, L., Malapani, C., Moore, H., and Hen, R. (2006). Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat. Neurosci. 9, 729–731.
Olson, A. K., Eadie, B. D., Ernst, C., and Christie, B. R. (2006). Environemental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 16, 250–260.
Raber, J., Rola, R., LeFevour, A., Morhardt, D., Curley, J., Mizumatsu, S., VandenBerg, S. R., and Fike, J. R. (2004). Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 162, 39–47.
Rola, R., Raber, J., Rizk, A., Otsuka, S., VandenBerg, S. R., Morhardt, D. R., and Fike, J. R. (2004). Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 188, 316–330.
Rossi, C., Angelucci, A., Costantin, L., Braschi, C., Mazzantini, M., Babbini, F., Fabbri, M. E., Tessarollo, L., Maffei, L., Berardi, N., and Caleo, M. (2006). Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 24, 1850–1856.
Sairanen, M., Lucas, G., Ernfors, P., Castren, M., and Castren, E. (2005). Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 25, 1089–1094.
Saxe, M. D., Battaglia, F., Wang, J. W., Malleret, G., David, D. J., Monckton, J. E., Garcia, A. D., Sofroniew, M. V., Kandel, E. R., Santarelli, L., Hen, R., and Drew, M. R. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 103, 17501–17506.
Scharfman, H., Goodman, J., Macleod, A., Phani, S., Antonelli, C., and Croll, S. (2005). Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 192, 348–356.
Shors, T. J., Townsend, D. A., Zhao, M., Kozorovitskiy, Y., and Gould, E. (2002). Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584.
Siegmund, A., Langnaese, K., and Wotjak, C. T. (2005). Differences in extinction of conditioned fear in C57BL/6 substrains are unrelated to expression of alpha-synuclein. Behav. Brain Res. 157, 291–298.
Snyder, J. S., Glover, L. R., Sanzone, K. M., Kamhi, J. F., and Cameron, H. A. (2009). The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus 19, 898–906.
Snyder, J. S., Hong, N. S., McDonald, R. J., and Wojtowicz, J. M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852.
Stranahan, A. M., Khalil, D., and Gould, E. (2006). Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 9, 526–533.
Tolwani, R. J., Buckmaster, P. S., Varma, S., Cosgaya, J. M., Wu, Y., Suri, C., and Shooter, E. M. (2002). BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience 114, 795–805.
van Praag, H., Kempermann, G., and Gage, F. H. (1999a). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270.
van Praag, H., Christie, B. R., Sejnowski, T. J., and Gage, F. H. (1999b). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U.S.A. 96, 13427–13431.
van Praag, H., Shubert, T., Zhao, C., and Gage, F. H. (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685.
Vaynman, S., Ying, Z., and Gomez-Pinilla, F. (2003). Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience 122, 647–657.
Vaynman, S., Ying, Z., and Gomez-Pinilla, F. (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 20, 2580–2590.
Warner-Schmidt, J. L., Madsen, T. M., and Duman, R. S. (2008). Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur. J. Neurosci. 27, 1485–1493.
Winocur, G., Wojtowicz, J. M., Sekeres, M., Snyder, J. S., and Wang, S. (2006). Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16, 296–304.
Wirth, M. J., Brun, A., Grabert, J., Patz, S., and Wahle, P. (2003). Accelerated dendritic development of rat cortical pyramidal cells and interneurons after biolistic transfection with BDNF and NT4/5. Development 130, 5827–5838.
Zhang, C. L., Zou, Y., He, W., Gage, F. H., and Evans, R. M. (2008). A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451, 1004–1007.
Zhao, C., Deng, W., and Gage, F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660.
Keywords: Ras, exercise, BDNF, object recognition, learning, memory
Citation: Lafenetre P, Leske O, Wahle P and Heumann R (2011) The beneficial effects of physical activity on impaired adult neurogenesis and cognitive performance. Front. Neurosci. 5:51. doi:10.3389/fnins.2011.00051
Received: 26 October 2010;
Paper pending published: 06 February 2011;
Accepted: 29 March 2011;
Published online: 12 April 2011.
Edited by:
John F. Cryan, University College Cork, IrelandReviewed by:
Henriette van Praag, National Institutes of Health, USAAine Kelly, The University of Dublin, Ireland
Copyright: © 2011 Lafenetre, Leske, Wahle and Heumann. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Dr. Pauline Lafenetre, University of Bordeaux-IPB, Laboratory of Nutrition and Integrative Neurobiology, Avenue des Facultés, Talence cedex, 33405, France, paulinelafenetre@yahoo.fr
