Differential effects of emotional information on interference task performance across the life span
- 1 Division of Psychiatry Research, Zucker Hillside Hospital, Glen Oaks, NY, USA
- 2 Department of Psychiatry, Duke University Medical Center, Durham, NC, USA
- 3 Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL, USA
- 4 Department of Psychiatry, Maryland Psychiatric Research Hospital, Baltimore, MD, USA
- 5 Division of Psychiatry Research, Zucker Hillside Hospital/Litwin Zucker Alzheimer’s Disease Center, Feinstein Institute/Albert Einstein College of Medicine, Glen Oaks, NY, USA
While functioning in multiple domains declines with age, emotional regulation appears to remain preserved in older adults. The Emotion Inhibition (Emotional Stroop) Test requires participants to name the ink color in which neutrally and emotionally valenced words are printed. It was employed in the current investigation as a measure of affective regulation in the context of an interference task in relation to age. Results demonstrated that while participants ranging from 20 to 50 years of age performed significantly worse on the emotion Stroop Inhibition relative to the neutral Stroop Inhibition condition, subjects over 60 years of age displayed the converse of this pattern, performing better on the emotion than the neutral condition, suggesting that they are less affected by the emotional impact of the positive and negative words used in the former condition. This pattern of age-related change in the ability to manage emotion may be related to blunting of affective signaling in limbic structures or, at the psychological level, focusing on emotional regulation.
Introduction
Multiple cognitive processes have been shown to decline with age, including tasks involving cognitive control such as the Stroop test. However, in contrast to these findings of cognitive changes, it has been demonstrated that emotional regulation may remain preserved or even be augmented in late life (Mather and Cartsensen, 2005). Older adults have been shown to be better able to sustain positive emotional states, as well as to avoid the occurrence of negative emotional states relative to younger adults (Carstensen et al., 2000). Older adults may also exhibit changes in the way they process or remember emotional information on a moment-to-moment basis in tasks involving affective regulation. For example, older adults demonstrate better memory for positive material rather than for neutral or negative information as compared to younger adults (Charles et al., 2003; Mather and Cartsensen, 2005; Mikels et al., 2005).
The Stroop test has been employed in multiple studies investigating the effects of age on cognitive control, particularly in relation to frontal lobe functions. Designed as an assessment of inhibition, the traditional Stroop task measures the ability to name the ink color in which a word is printed when it is incongruent with the meaning of the word (Stroop, 1935). Results demonstrate that older adults exhibit the greatest effect of such interference (Comalli et al., 1962; Cohn et al., 1984). A variant of the original Stroop interference paradigm, the emotional Stroop task presents subjects with neutral and emotionally laden words (i.e., murder) presented in different ink colors (Whalen et al., 1998), combining demands for affective regulation and cognitive control. In this paradigm, there are costs in accuracy and/or response time for the emotional interference and management. Functional neuroimaging studies have found that the task engages anterior cingulate regions often thought to monitor conflict (Peyron et al., 2000; Lagopoulos and Malhi, 2007; Park et al., 2008; Wingenfeld et al., 2009) or regulate emotion, e.g., the subgenual cingulate (Whalen et al., 1998). The amygdala, thought to underlie the processing of emotions and emotional material (Compton et al., 2003; Lagopoulos and Malhi, 2007), has also been shown to be activated by the emotional Stroop paradigm. Correspondingly, there is evidence that activation of the anterior cingulate cortex modulates activation in the amygdala (Etkin et al., 2006).
We elected to examine emotional regulation and control as a function of age in a large sample of healthy controls across a wide age range using a new, simplified version of the emotional Stroop (i.e., the Emotion Inhibition Test) that controls for speed and general interference. Two prior studies examining age effects on the emotional Stroop yielded conflicting results, used different methodologies than those used by us, and had smaller samples (see section Discussion). For our study, we predicted that younger subjects would process information more quickly (i.e., read more words) in the neutral inhibition condition compared to the emotion inhibition condition. Contrastingly, we expected that the older adults would perform equally on the neutral and emotional trials. Finally, we expected that these results would be independent of general slowing in the older groups.
Materials and Methods
Subjects
Four hundred and four participants were included in this study. Subjects were recruited using a random digit dialing method with telephone numbers generated by Survey Sampling, Inc. in Fairfield, CT. Potential participants were provided with an overview of the study and were then offered the option of discontinuing the call if they were not interested. Based on self-report, subjects were screened on the basis of the exclusionary criteria described below. For those subjects who met the basic enrollment criteria, a more complete eligibility interview was conducted. All participants completed the written informed consent process. The study was approved through Duke University’s IRB. A comprehensive review of the subject identification and recruitment strategy is provided in the original study published by Keefe et al. (2008).
All eligible subjects were screened using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and were asked targeted questions about substance use for life and for the past month. In regard to the use of drugs or alcohol, exclusion criteria included (a) persistent alcohol abuse (i.e., more than four drinks per day or habitual binging for longer than 10 years), (b) prolonged drug use (i.e., under the influence for more than 50% of the waking day or regular binging for longer than 10 years), (c) routine use of illegal drugs, or (d) withdrawal from substances.
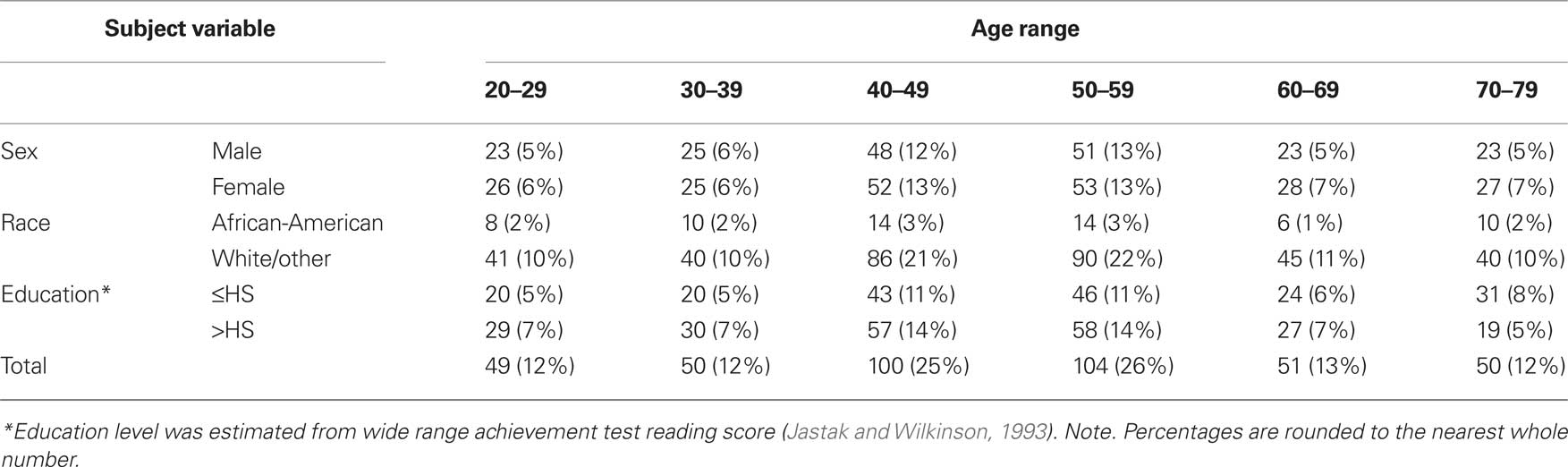
The demographics of the sample are displayed in Table 1 and are representative of the US population.
For a more thorough description of the participants, please refer to Keefe et al. (2008). For the sample as a whole, the mean age was 49.6 ± 14.93 years. As shown in Table 1, the participants were divided into six groups based on decade of life with the 40s and 50s having the greatest number of subjects. The age groups did not differ in terms of education (in grades) (F5,398 = 0.49, p = 0.78). Mean education ranged by age group from 14.1 to 14.7 grades.
Assessment Procedures
All tests were administered in person by trained and experienced research assistants. For a comprehensive list of the measures used in the original study, refer to Keefe et al. (2008). All data presented below were collected in relation to the parent study, but have not been previously reported. Following is a description of those tests that are pertinent to the present study:
General cognitive functioning
All participants completed the brief assessment of cognition in schizophrenia (BACS), a short but comprehensive screen of neuropsychological abilities including verbal memory, working memory, motor speed, processing speed, executive functions, and attention. The normative data for this instrument are presented in Keefe et al. (2008). As reported in the original article, results revealed effects of age and education on cognitive abilities, with lower scores being associated with later age and fewer years of education. Having controlled for age and education, gender was shown to account for only 2% of the variance in BACS scores.
Emotion inhibition (emotional Stroop) test
The task had four conditions: (1) Color Naming in which subjects were administered a rapid color-naming task requiring them to name the color (blue, red, green) of as many patches of color as possible in 30s; (2) Word Reading involved rapid word reading in which subjects were asked to read as many emotionally neutral words (e.g., bench, paper, jelly) printed in black ink as possible in 30s; (3) the Emotion Inhibition Condition required subjects to quickly name the color of the ink in which negative and positive emotional words (e.g., success, fire, rage) were printed; (4) the Neutral Inhibition Condition required subjects to quickly name the color of the ink in which neutrally valenced words (e.g., journal, lamb, bus) were printed. The score for the latter two conditions was the number of colors named in 30s. For the emotional words, frequency ranged from 1 to 274, with a median standard frequency index (SFI) of 52.95 (Bradley and Lang, 1999). The valence of negative words was under 3.32 (range 3.31–2.1) and the valence of the positive words was greater than 7.15 (range 7.15–8.37). Arousal level was 6.37 (range 6.07–8.77). In regard to the neutral words, the frequency ranged from 3 to 255, with a median SFI of 56.6. The valence median for neutral words was 5.24 (range 4.39–5.89) and the arousal median was 3.74 (range 2.82–4.08). Words in all conditions contained no more than two syllables. In order to minimize the effects of previous conditions on performance, the conditions were always administered in this order: Color Naming, Neutral Inhibition, Emotion Inhibition, and Word Reading.
Results
The mean raw scores for each age group across the four conditions of the Emotion Inhibition Test are presented in Figure 1.
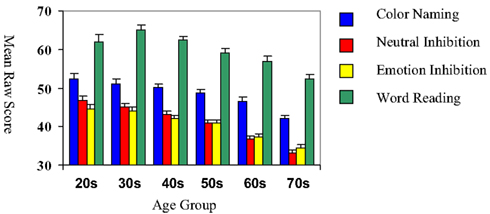
Figure 1. Performance on the emotional inhibition test across age group. Colored bars represent means for performance in each condition of the emotional inhibition; error bars reflect standard error of the means. Age groups are on the x axis. Note general declines in performance with age, but relatively better performance on the Emotional Inhibition, as compared to the Neutral Inhibition condition, with advancing age.
A repeated measures analysis of variance (ANOVA) revealed a significant main effect of age group on the number of correct responses for the two interference conditions, neutral and emotion, (F = 4.88, p = 0.03) as well as a significant interaction of age group and interference condition (F = 6.43, p < 0.0001). Overall, older adults (i.e., ages 60–79) had fewer correct responses on the emotional and neutral interference trials relative to younger adults (i.e., ages 20–49). The response patterns of the older groups demonstrated better performance in affective than neutral inhibition; the younger groups exhibited a greater effect of the emotional material, performing significantly more poorly on the affective relative to the neutral condition.
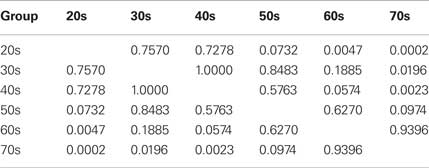
An analysis of covariance (ANCOVA) using the difference score between the number of correct responses on the emotion and neutral inhibition conditions as the dependent variable revealed significant differences across age groups (F = 6.41, p < 0.0001). In order to control for possible variability in processing speed, the color-naming and word-reading scores were used as covariates in this analysis. Results remained similar to the repeated measures analysis reported above. Post hoc comparisons between age groups were conducted using the Tukey’s test in order to control for multiple comparisons (see Table 2).
Table 2 shows the Tukey’s p-value of pairwise comparisons between age groups on the difference score between emotional and neutral inhibition conditions. In general, participants in their 20s, 30s, 40s did not differ from each other. Similarly, participants in their 60s and 70s did not differ from each other. However and critically, the younger age groups differed significantly from these older age groups (all ps < 0.05 with a single exception).
These raw difference scores are displayed graphically in Figure 2. While younger adults (in the 20-, 30-, and 40-year age groups) performed worse on the emotional inhibition condition relative to the neutral inhibition condition, this pattern was reversed in older adults in the 60 and 70-year age groups. In other words, the younger adults were more impacted by the emotional stimuli, as compared to older adults.
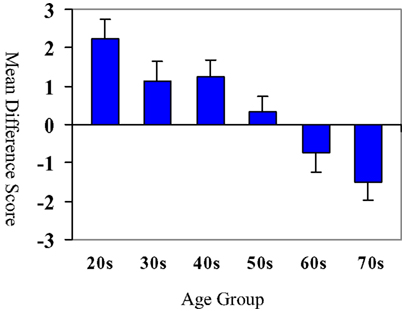
Figure 2. Difference score by age group: neutral inhibition–emotion inhibition. Bars represent means of neutral inhibition–emotional inhibition difference score (i.e., the difference between the number of correct responses on the neutral inhibition task and the emotional inhibition task). Age groups are on the x axis. Note the change in sign in the difference score in the 60- and 70-year-old age groups as this demonstrates that older participants had more correct responses on the emotional inhibition task relative to the neutral inhibition task This pattern of performance is indicative of a decreased susceptibility to emotional stimuli among older adults and increased susceptibility to emotional stimuli in younger adults.
To summarize this set of analyses, individuals in the 20s, 30s, and 40s age groups did not differ from each other (all ps > 0.70) and performed better in neutral than affective. Individuals in the 60s and 70s age groups did not differ from each other (p > 0.90) and performed better on affective than neutral conditions. Additionally the 60 and 70 age groups performed significantly differently when contrasted with the 20s, 30s, and 40s age groups in pairwise contrasts (all ps < 0.05 with a single exception). As seen in Figure 2, subjects in the 20s, 30s, and 40s age groups showed a difference score that was positive. Interestingly, subjects in the 60s and 70s age groups showed a reversal of sign, such that the difference scores were now negative. Of course, this is consistent with the age group x task condition (affective/neutral) interaction effect by repeated measures ANOVA (below). Subjects in the 50s age group appeared to be in a transitional phase, as difference scores were near 0.
Finally, a repeated measures ANOVA showed a main effect of age group (F = 19.75, p < 0.0001), but no main effect of gender (F = 5.45, p = 0.02) on performance across the four conditions of the Emotion Inhibition Test. Furthermore, the interaction between age group and gender was not significant (F = 0.69, p = 0.63). These results suggest that gender does not impact upon performance on the Emotion Inhibition Test task in normal control subjects.
Discussion
Based on the above results, the performances of individuals later in life, regardless of gender, were less susceptible to the effects of emotional information during a cognitive conflict task than younger adults. Not unexpectedly, subjects in their 50s, 60s, and 70s had significantly fewer overall correct responses across the word reading and color-naming conditions. However, and strikingly, while participants ranging from 20 to 50 years of age performed significantly worse on the affective Stroop relative to the neutral Inhibition condition, subjects over 60 years of age displayed the converse of this pattern, performing better on the affective than the neutral condition. This suggested that they were less affected by the emotional impact of the positive and negative words used in the former condition.
We believe that our study is perhaps the first to unequivocally demonstrate increased ability to manage emotional stimuli (in the context of cognitive incongruity and inhibition) in older versus younger subjects. It is important to highlight the factors that distinguish the present study from those that previously either found negative aging effects in the context of an auditory Stroop (Wurm et al., 2004) or positive aging effects (shown as a reduced carry-over effect from negative to neutral words in mixed blocks only) on the regulation of an emotional Stroop paradigm (Ashley and Swick, 2009). The present study utilized the largest sample of participants. Furthermore, to allow for the most parsimonious demonstration of the effect of age on emotional regulation, the present study only presented affective, and neutral interference stimuli visually, in pure blocks of neutral and emotionally valenced words, which perhaps amplified response styles to the different types of stimuli (emotional or neutral) and carefully controlled for general issues of speed.
Stroop interference may be the result of conflict monitoring, response competition, etc. Our study was not designed to adjudicate among them (see also Whalen et al., 1998). In our study the two main conditions (emotional interference/inhibition versus neutral interference/inhibition) both involved a Stroop effect. Rather, they differed only in the affective valence of the stimuli themselves, which exacted a cost in younger individuals and produced a benefit in older individuals.
This study has several possible limitations. The order of the tests was not counterbalanced. However, in large-scale normative and clinical studies this is generally not practical. Moreover, in our estimation it is unlikely that age, test, and test order would have interacted to yield the current predicted results. Nevertheless, complex carry-over effects cannot be completely ruled out. We also did not correct for general reading or composite cognition, because we had internal control conditions (word reading, color naming) directly relevant to the task and because we had within subject neutral and affective interference conditions. Last, we mixed positive and negatively valenced words in our affective condition, precluding more refined positive/negative analysis, but perhaps amplifying an “affective signal.”
The question remains as to why emotional regulation appears to be preserved, or at least less disrupted in later life in terms of accuracy and response time costs, as exemplified by the results of this study. One potential neurobiological explanation involves amygdala processing, as this structure has been shown to play an important role in affective signaling. Previous functional imaging studies examining facial processing have consistently reported decreased activation in the amygdala in older adults in response to negative facial expressions compared to younger participants (Iidaka et al., 2002; Calder et al., 2003; Gunning-Dixon et al., 2003). Amygdala activation has been found in some studies in the appraisal of positive emotional information, and signaling has also been shown to diminish with age (Williams et al., 2006; Suslow et al., 2010). Based on the imaging results, it appears likely that older adults demonstrate a diminished response to affective stimuli, thereby resulting in decreased level of conflict between task relevant and task irrelevant processing. Of course, how older individuals use the results of such affective regulations to influence their decision making is an important issue for additional research.
At the psychological level, older individuals may implement efforts to manage emotion or not become distracted by it so as to make more adaptive or more objective decisions because they perceive that have limited time before death. This may, at least in part, relate to the socioemotional selectivity theory which states that individuals adjust their goals to be more focused on the regulation of emotion rather than the open-ended pursuit of information and new experiences as they age, perceiving time as more finite (Carstensen et al., 1999). It is likely that the neurobiological and psychological explanations are not mutually exclusive.
In summary, our results suggest that the Emotion Inhibition Test is a practical behavioral method for examining the process of aging in the older adult population. As the measure is brief, easy to administer, and cost effective, it makes for a useful tool for future studies of cognitive and affective regulation during aging.
Disclosure Statement
Drs. Gold, Goldberg, Harvey, and Keefe receive royalties for use of the BAC in clinical trials.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
The funding for this study was provided by Zucker Hillside Hospital.
References
Ashley, V., and Swick, D. (2009). Consequences of emotional stimuli: age differences on pure and mixed blocks of the emotional Stroop. Behav. Brain Funct. 5, 14.
Bradley, M. M., and Lang, P. J. (1999). Affective Norms for English Words (ANEW): Stimuli, Instruction Manual and Affective Ratings. Technical Report C-1. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida.
Calder, A. J., Keane, J., Manly, T., Sprengelmeyer, R., Scott, S., Nimmo-Smith, I., and Young, A. W. (2003). Facial expression recognition across the adult lifespan. Neuropsychologia 41, 195–202.
Carstensen, L. L., Isaacowitz, D. M., and Charles, S. T. (1999). Taking time seriously: a theory of socioemotional selectivity. Am. Psychol. 54, 165–181.
Carstensen, L. L., Pasupathi, M., Mayr, U., and Nesselroade, J. R. (2000). Emotional experience in everyday life across the adult life span. J. Pers. Soc. Psychol. 79, 644–655.
Charles, S. T., Mather, M., and Carstensen, L. L. (2003). Aging and emotional memory: the forgettable nature of negative images for older adults. J. Exp. Psychol. Gen. 132, 310–324.
Cohn, N. B., Dustman, R. E., and Bradford, D. C. (1984). Age-related decrements in Stroop color test performance. J. Clin. Psychol. 40, 1244–1250.
Comalli, P. E., Jr., Wapner, S., and Werner, H. (1962). Interference effects of Stroop color-word test in childhood, adulthood, and aging. J. Genet. Psychol. 100, 47–53.
Compton, R. J., Banich, M. T., Mohanty, A., Milham, M. P., Herrington, J., Miller, G. A., Scalf, P. E., Webb, A., and Heller, W. (2003). Paying attention to emotion: an fMRI investigation of cognitive and emotional Stroop tasks. Cogn. Affect. Behav. Neurosci. 3, 81–96.
Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R., and Hirsch, J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51, 871–882.
Gunning-Dixon, F. M., Gur, R. C., Perkins, A. C., Schroeder, L., Turner, T., Turetsky, B. I., Chan, R. M., Loughead, J. W., Alsop, D. C., Maldjian, J., and Gur, R. E. (2003). Age-related differences in brain activation during emotional face processing. Neurobiol. Aging 24, 285–295.
Iidaka, T., Okada, T., Murata, T., Omori, M., Kosaka, H., Sadato, N., and Yonekura, Y. (2002). Age-related differences in medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus 12, 352–362.
Jastak, S., and Wilkinson, G. S. (1993). Wide Range Achievement Test-Revised 3. Jastak Associates, Wilmington, DE.
Keefe, R. S. E., Harvey, P. D., Goldberg, T. E., Gold, J. M., Walker, T. M., Kennel, C., and Hawkins, K. (2008). Norms and standardization of the brief assessment of cognition in schizophrenia (BACS). Schizophr. Res. 102, 108–115.
Lagopoulos, J., and Malhi, G. S. (2007). A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Neuroreport 8, 1583–1587.
Mather, M., and Cartsensen, L. L. (2005). Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn. Neurosci. 9, 496–502.
Mikels, J. A., Larkin, G. R., Reuter-Lorenz, P. A., and Carstensen, L. L. (2005). Divergent trajectories in the aging mind: changes in working memory for affective versus visual information with age. Psychol. Aging 20, 542–553.
Park, I. H., Park, H. J., Chun, J. W., Kim, E. Y., and Kim, J. J. (2008). Dysfunctional modulation of emotional interference in the medial prefrontal cortex in patients with schizophrenia. Neurosci. Lett. 440, 119–124.
Peyron, R., Laurent, B., and García-Larrea, L. (2000). Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol. Clin. 30, 263–288.
Suslow, T., Konrad, C., Kugel, H., Rumstadt, D., Zwitserlood, P., Schöning, Ohrmann, P., Bauer, J., Pyka, M., Kersting, A., Arolt, V., Heindel, W., and Dannlowski, U. (2010). Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol. Psychiatry 67, 155–160.
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662.
Whalen, P. J., Bush, G., McNally, R. J., Wilhelm, S., McInerney, S. C., Jenike, M. A., and Rauch, S. L. (1998). The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulated affective division. Biol. Psychiatry 44, 1219–1228.
Williams, L. M., Brown, K. J., Palmer, D., Liddell, B. J., Kemp, A. H., Olivieri, G., Peduto, A., and Gordon, E. (2006). The mellow years?: neural basis of improving emotional stability over age. J. Neurosci. 26, 6422–6430.
Wingenfeld, K., Rullkoetter, N., Mensebach, C., Beblo, T., Mertens, M., Kreisel, S., Toepper, M., Driessen, M., and Woermann, F. G. (2009). Neural correlates of the individual emotional Stroop in borderline personality disorder. Psychoneuroendocrinology 34, 571–586.
Keywords: emotional Stroop paradigm, emotional regulation, cognitive control, aging, amygdala, anterior cingulate
Citation: LaMonica HM, Keefe RSE, Harvey PD, Gold JM and Goldberg TE (2010) Differential effects of emotional information on interference task performance across the life span. Front. Ag. Neurosci. 2:141. doi: 10.3389/fnagi.2010.00141
Received: 11 June 2010;
Paper pending published: 12 June 2010;
Accepted: 08 September 2010;
Published online: 30 September 2010.
Edited by:
Jeffrey Kaye, Oregon Health & Science University, USAReviewed by:
Paul Whalen, Darmouth College, USASusan M. Resnick, National Institute on Aging, USA
Izhak Shafran, Oregon Health & Science University, USA
Copyright: © 2010 LaMonica, Keefe, Harvey, Gold and Goldberg. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Terry E. Goldberg, Division of Psychiatry Research, Zucker Hillside Hospital/Litwin Zucker Alzheimer’s Disease Center, Feinstein Institute/AECOM, Glen Oaks, NY, USA. e-mail: tgoldber@nshs.edu