A Sustainable and Efficient Synthesis of Benzyl Phosphonates Using PEG/KI Catalytic System
- 1Department of Chemistry, Institute of Chemical Technology, Mumbai, India
- 2Department of Chemistry, SIES College of Arts, Science and Commerce, Mumbai, India
- 3Department of Chemistry, B.N. Bandodkar College of Science, Mumbai, India
- 4Department of Chemical Engineering, Motilal Nehru National Institute of Technology, Allahabad, India
- 5Department of Physical Chemistry, Faculty of Science, Regional Centre of Advanced Technologies and Materials, Palacky University, Olomouc, Czech Republic
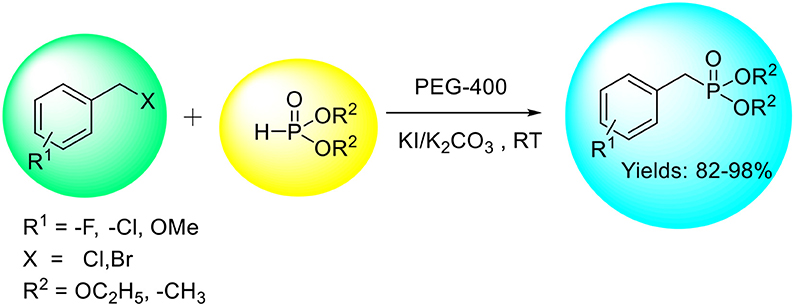
An efficient and expedient protocol for the synthesis of benzyl phosphonates using KI/K2CO3 as a catalytic system and PEG-400 as benign solvent has been developed. The reaction proceeds smoothly at room temperature achieving excellent selectivity and yield of the corresponding products. The combination of PEG-400, KI, and K2CO3 in this reaction avoids the need of volatile/toxic organic solvents and reactive alkali metals or metal nanoparticles/hydrides. We believe that this benign combination (PEG-400 and KI) could be used for other related organic transformations.
Introduction
Compounds containing C-P bonds are an attractive synthetic targets because of their significant applications as isosteric analogs of phosphate esters (Engel, 1977; Huang and Chen, 2000; Manabe and Kobayashi, 2000), target-specific modulators for biological processes (Engel, 1992), phosphonopeptides (Kafarski and Lejczak, 1991), amino acid analogs (Rushing and Hammer, 2001; Fredriksen and Amedjkouh, 2016), and as pro-drugs (Krise and Stella, 1996). Esters of phosphonic acids are key and important intermediates in a variety of synthetically important reactions such as Wadsworth-Emmons reactions (Quin, 2000), and they also act as chelating agents for many imperative metals (Anderson et al., 2002). Although phosphorus compounds containing the C-P bond are not particularly abundant in nature (Kittredge and Roberts, 1969), their formation still remains a formidable challenge.
Conventional methods for the synthesis of organophosphorus compounds are Michaelis-Arbuzov (Bhattacharya and Thyagarajan, 1981; Anna and Artur, 2015), and Michaelis-Becker reactions (Meisters and Swan, 1965). Michaelis-Arbuzov method involves the formation of a phosphonium intermediate through the nucleophilic addition of the phosphorus lone pair to the alkyl halide to form a new alkyl halide and the target product alkyl phosphonate. These conventional methods suffer from major drawbacks, when this newly formed alkyl halide is more reactive or less volatile than the initial alkyl halide used thus resulting in a mixture of phosphorylated products (Saady et al., 1995a,b). This reaction also requires high temperature, particularly for unreactive halides. In Michealis-Becker reaction, an alkali metal salt of dialkyl phosphate reacts with an alkyl halide under milder reaction conditions but requires strong bases (Kers et al., 1997). Sometimes a mixture of products are formed by single electron transfer (SET) mechanism due to the high reactivity of phosphate anion generated with the substrates having pseudo halide character (Michalski et al., 1991; Engel, 1992; Witt and Rachon, 1996). The reaction can also be carried out under phase transfer catalyzed conditions but the limitations of these methods are not clearly explained (Kem et al., 1981; Shi et al., 2000; Tomilov et al., 2001). Therefore, to alleviate these problems, other methods have been investigated (Lavén and Stawinski, 2009; Takahashi et al., 2009; Rajeshwaran et al., 2011; Bloomfield and Herzon, 2012; Xu et al., 2013; Wang et al., 2014) Hence, there is scope to develop new methodology or to improve the previous methods to form the P-C bond.
Polyethylene glycol (PEG) is an environmentally benign alternative reaction medium in synthetic chemistry (Chen et al., 2005), and used in various substitution, oxidation and reduction reactions. PEG and its monomethyl ethers have been widely used as phase transfer catalyst (PTC) in many organic reactions (Timko et al., 1977; Dickerson et al., 2002; Chandrasekhar et al., 2003), due to their unique advantages of high thermal stability, negligible vapor pressure, low cost, easy availability and recyclability. The high water miscibility of PEG facilitates its separation from reaction products when used as a reaction medium.
Experimental Methods
Reaction Procedure
To a stirred mixture of benzyl halide (1 mmol), dialkyl phosphite (1 mmol), K2CO3 (2 mmol), KI (0.3 mmol) and PEG-400 (0.5 g) was added. The reaction mixture was stirred at room temperature for 6 h. The progress of the reaction was monitored by TLC. After completion of reaction the product formed was then extracted with diethyl ether (2 × 10 mL). The obtained residual oil was further purified by using column chromatography (petroleum ether/ethyl acetate 10%).
Results and Discussion
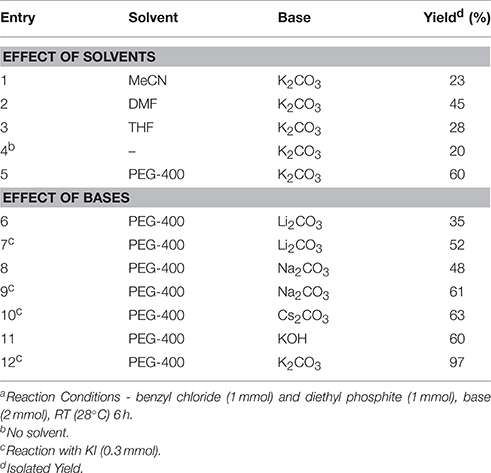
In continuation of our efforts for synthesis of organic compounds using sustainable protocols (Gawande et al., 2013a,b,c,d, 2014a,b,c; Kale et al., 2013; Sharma et al., 2015), in this work, we have successfully developed a mild and ecofriendly synthesis of benzyl phosphonates using PEG/KI as a catalytic system. In this protocol, PEG not only acts as a reaction medium but also as a PTC. We have used KI for in situ formation of benzyl iodide since it is inexpensive, nontoxic and readily available reagent. The use of KI avoids direct use of expensive benzyl iodide. Initially, we chose benzyl chloride (1 mmol) and diethyl phosphate (1 mmol) as model substrates to establish the optimum conditions for the reaction (Scheme 1).
We examined the effect of various solvents such as MeCN, DMF, THF, and PEG-400 at room temperature by adding K2CO3 as a base in the reaction. The reaction was found to be solvent dependent giving the best result in PEG-400 (Table 1, entry 12). PEG enhances the reactivity of the inorganic base by chelation of the countercation. To further improve the efficiency of this green synthetic approach, various carbonates and hydroxides of other alkali and alkaline earth metals were applied as bases to promote this transformation in PEG. Both cesium carbonate and KOH gave comparable yields of corresponding phosphonates along with benzyl alcohol as the side product and hence, we decided to use powdered anhydrous K2CO3. To improve the catalytic performance in terms of yield, KI was tested as an additive. In this case the addition of KI resulted in a significant improvement in the percent yield of the phosphonates. 0.3 eq. of KI and 2 eq. of K2CO3 were found to be the optimum amounts giving best results. The reaction conditions were then finally established (Table 1, Entry 12).
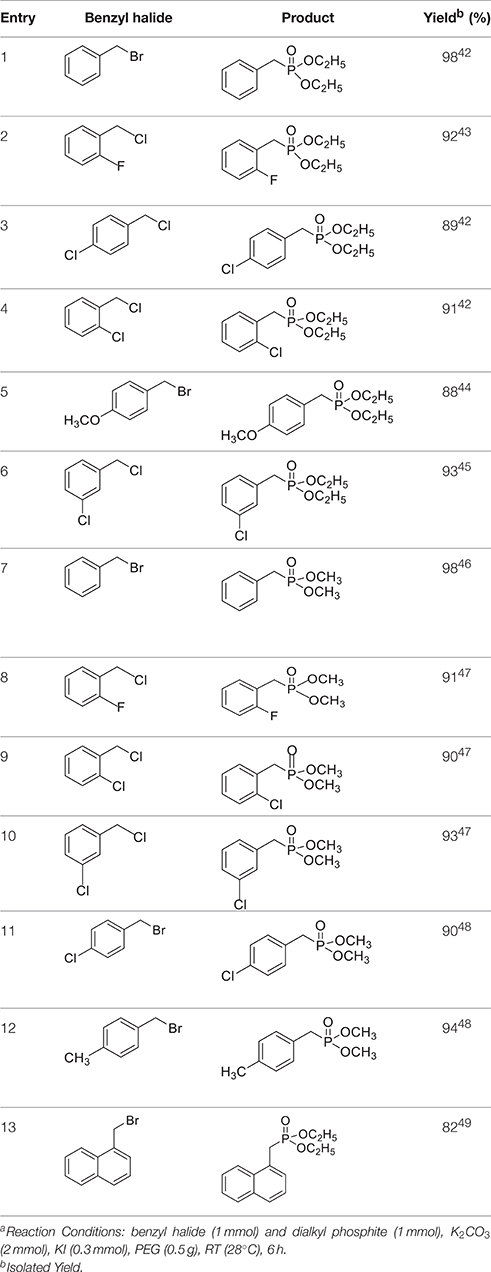
Once the optimum conditions have been established, a range of substituted benzyl halides (1 mmol) were reacted with various dialkyl phosphites (1 mmol) in the presence of KI (0.3 mmol) and anhydrous powdered K2CO3 (2 mmol) using PEG-400 as a reaction medium. The reaction mixture was stirred at room temperature for 6 h. Formation of the corresponding benzyl phosphonates occurs with good to excellent yields of the desired products. The versatility of this process was demonstrated with respect to various electron donating and electron withdrawing benzyl halides (Table 2).
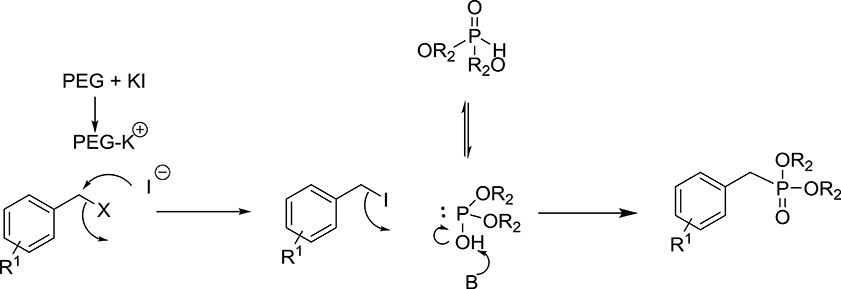
On the basis of experimental results and the literature, the possible mechanism for the formation of substituted benzyl dialkyl phosphonate is likely to take place in two steps (Scheme 2). The initial step, formation of benzyl iodide is likely to occur by Fenkelstain reaction, in which the chloride or bromide ion is replaced by the iodide. The PEG-400 may be facilitating this step by assisting the dissociation of KI. The possible role of PEG is to activate the anion by forming a complex with the cation similar to crown ether. This means that PEG enhances the nucleophilicity of the iodide ions and facilitates the conversion of benzyl chloride/bromide into benzyl iodide. To check the formation of benzyl iodide in the course of the reaction, we carried out the reaction between KI and benzyl chloride in the absence of K2CO3 and phosphite in PEG-400 and observed that benzyl iodide is formed in good yield which was further confirmed by GC and GCMS analysis (see Supplementary Material). In the second step of the reaction, the nucleophilic displacement of iodide takes place by the dialkyl phosphite to give the corresponding phosphonate.
In conclusion, we have developed a simple, efficient and sustainable protocol for the synthesis of benzyl phosphonates from benzyl halides using KI/K2CO3 in PEG-400. PEG-400 may be used as solvent for the Fenkelstein reaction instead of volatile acetone. The advantages include broad application scope, mild reaction conditions, excellent yields, simple operation, cost effectiveness and environmental friendliness which make this protocol a value addition to the existing methods in the synthesis of benzyl phosphonates.
Author Contributions
SD, SKal, and SKah worked on the experimental parts, GA, AS helped writing and MG is the supervisor of this research work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SD is thankful to Indira Gandhi Centre for Atomic Research (IGCAR) for financial assistance. The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2015073, and support from the Ministry of Education, Youth and Sports of the Czech Republic—project No. LO1305.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fchem.2016.00035
References
Anderson, P. M., Wiseman, G. A., Dispenzieri, A., Arndt, C. A. S., Hartmann, L. C., Smithson, W. A., et al. (2002). High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J. Clin. Oncol. 20, 189–196. doi: 10.1200/JCO.20.1.189
Anna, D., and Artur, M. (2015). Catalytic and MW-assisted michaelis-arbuzov reactions. Curr. Green Chem. 2, 223–236. doi: 10.2174/2213346102666150128195001
Bhattacharya, A. K., and Thyagarajan, G. (1981). Michaelis-Arbuzov rearrangement. Chem. Rev. 81, 415–430. doi: 10.1021/cr00044a004
Bloomfield, A. J., and Herzon, S. B. (2012). Room temperature, palladium-mediated P–arylation of secondary phosphine oxides. Org. Lett. 14, 4370–4373. doi: 10.1021/ol301831k
Chandrasekhar, S., Narsihmulu, Ch., Sultana, S. S., and Reddy, N. R. (2003). Osmium tetroxide in poly(ethylene glycol) (PEG): a recyclable reaction medium for rapid asymmetric dihydroxylation under Sharpless conditions. Chem. Commun. 1716–1717. doi: 10.1039/b305154B
Chen, J., Spear, S. K., Huddleston, J. G., and Rogers, R. D. (2005). Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 7, 64–82. doi: 10.1039/b413546f
Dickerson, T. J., Reed, N. N., and Janda, K. D. (2002). Soluble polymers as scaffolds for recoverable catalysts and reagents. Chem. Rev. 102, 3325–3344. doi: 10.1021/cr010335e
Engel, R. (1977). Phosphonates as analogues of natural phosphates. Chem. Rev. 77, 349–367. doi: 10.1021/cr60307a003
Fredriksen, K. A., and Amedjkouh, M. (2016). Investigation of reactive intermediates and reaction pathways in the coupling agent mediated phos-phonamidation reaction. Eur. J. Org. Chem. 2016, 474–482. doi: 10.1002/ejoc.201501244
Gawande, M. B., Bonifácio, V. D. B., Luque, R., Branco, P. S., and Varma, R. S. (2013a). Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 42, 5522–5551. doi: 10.1039/c3cs60025d
Gawande, M. B., Bonifácio, V. D. B., Luque, R., Branco, P. S., and Varma, R. S. (2014a). Solvent-free and catalysts-free chemistry: a benign pathway to sustainability. ChemSusChem. 7, 24–44. doi: 10.1002/cssc.201300485
Gawande, M. B., Branco, P. S., and Varma, R. S. (2013b). Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 42, 3371–3393. doi: 10.1039/c3cs35480f
Gawande, M. B., Luque, R., and Zboril, R. (2014b). The rise of magnetically recyclable nanocatalysts. ChemCatChem. 6, 3312–3313. doi: 10.1002/cctc.201402663
Gawande, M. B., Rathi, A. K., Branco, P. S., and Varma, R. S. (2013c). Sustainable utility of magnetically recyclable nano-catalysts in water: applications in organic synthesis. Appl. Sci. 3, 656–674. doi: 10.3390/app3040656
Gawande, M. B., Rathi, A. K., Nogueira, I. D., Varma, R. S., and Branco, P. S. (2013d). Magnetite-supported sulfonic acid: a retrievable nanocatalyst for the Ritter reaction and multicomponent reactions. Green Chem. 15, 1895–1899. doi: 10.1039/c3gc40457a
Gawande, M. B., Shelke, S. N., Zboril, R., and Varma, R. S. (2014c). Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 47, 1338–1348. doi: 10.1021/ar400309b
Huang, J., and Chen, R. (2000). An overview of recent advances on the synthesis and biological activity of α-aminophosphonic acid derivatives. Heteroatom Chem. 11, 480–492. doi: 10.1002/1098-1071(2000)11:7<480::AID-HC6>3.0.CO;2-J
Kafarski, P., and Lejczak, B. (1991). Biological activity of aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 63, 193–215. doi: 10.1080/10426509108029443
Kale, S. R., Kahandal, S. S., Burange, A. S., Gawande, M. B., and Jayaram, R. V. (2013). A benign synthesis of 2-amino-4H-chromene in aqueous medium using hydrotalcite (HT) as a heterogeneous base catalyst. Catal. Sci. Technol. 3, 2050–2056. doi: 10.1039/c3cy20856g
Kem, K. M., Nguyen, N. V., and Cross, D. J. (1981). Phase-transfer-catalyzed michaelis-becker reaction. J. Org. Chem. 46, 5188–5192. doi: 10.1021/jo00338a025
Kers, A., Stawiński, J., Dembkowski, L., and Kraszewski, A. (1997). Aryl H-phosphonates. 7. Studies on the formation of phosphorus-carbon bond in the reaction of trityl and benzyl halides with dialkyl and diphenyl H-phosphonates. Tetrahedron 53, 12691–12698. doi: 10.1016/S0040-4020(97)00790-4
Kittredge, J. S., and Roberts, E. (1969). A carbon-phosphorus bond in nature. Science 164, 37–42. doi: 10.1126/science.164.3875.37
Krise, J. P., and Stella, V. J. (1996). Prodrugs of phosphates, phosphonates, and phosphinates. Adv. Drug Delivery Rev. 19, 287–310. doi: 10.1016/0169-409X(95)00111-J
Lavén, G., and Stawinski, J. (2009). Palladium(0)-catalyzed benzylation of H-phosphonate diesters: an efficient entry to benzylphosphonates. Synlett 2009, 225–228. doi: 10.1055/s-0028-1087522
Manabe, K., and Kobayashi, S. (2000). Facile synthesis of α-amino phosphonates in water using a Lewis acid—surfactant-combined catalyst. Chem. Commun. 669–670. doi: 10.1039/B000319K
Meisters, A., and Swan, J. (1965). Organophosphorus compounds. VI. The ‘abnormal’ Michaelis-Becker reaction. Diethyl 1-phenylepoxyethylphosphonate and diethyl 1-phenylvinylphosphate from diethyl phosphonate and phenacyl chloride. Australian J. Chem. 18, 168–172. doi: 10.1071/CH9650168
Michalski, J., Skowronska, A., and Łopusinski, A. (1991). New chemistry and stereochemistry of organophosphorus pseudohalogens. Phosphorus Sulfur Silicon Relat. Elem. 58, 61–88. doi: 10.1080/10426509108040626
Rajeshwaran, G. G., Nandakumar, M., Sureshbabu, R., and Mohanakrishnan, A. K. (2011). Lewis acid-mediated michaelis−arbuzov reaction at room temperature: a facile preparation of arylmethyl/heteroarylmethyl phosphonates. Org. Lett. 13, 1270–1273. doi: 10.1021/ol1029436
Rushing, S. D., and Hammer, R. P. (2001). Synthesis of phosphonamide and thiophosphonamide dipeptides. J. Am. Chem. Soc. 123, 4861–4862. doi: 10.1021/ja015632f
Saady, M., Lebeau, L., and Mioskowski, C. (1995a). Synthesis of di- and triphosphate ester analogs via a modified Michaelis-Arbuzov reaction. Tetrahedron Lett. 36, 5183–5186. doi: 10.1016/00404-0399(50)10097-
Saady, M., Lebeau, L., and Mioskowski, C. (1995b). First Use of Benzyl Phosphites in the Michaelis-Arbuzov Reaction synthesis of mono-, Di-, and triphosphate analogs. Helvetica Chim. Acta 78, 670–678. doi: 10.1002/hlca.19950780314
Sharma, R. K., Sharma, S., Dutta, S., Zboril, R., and Gawande, M. B. (2015). Silica-nanosphere-based organic-inorganic hybrid nanomaterials: synthesis, functionalization and applications in catalysis. Green Chem. 17, 3207–3230. doi: 10.1039/C5GC00381D
Shi, D.-Q., Wang, Y.-M., and Chen, R.-Y. (2000). Synthesis and stereoselectivity of a new type of unsaturated phosphonates. Heteroatom Chem. 11, 261–266. doi: 10.1002/1098-1071(2000)11:4<261::AID-HC3>3.0.CO;2-1
Takahashi, H., Inagaki, S., Yoshii, N., Gao, F., Nishihara, Y., and Takagi, K. (2009). Rh-catalyzed negishi alkyl-aryl cross-coupling leading to α- or β-phosphoryl-substituted alkylarenes. J. Org. Chem. 74, 2794–2797. doi: 10.1021/jo900142b
Timko, J. M., Moore, S. S., Walba, D. M., Hiberty, P. C., and Cram, D. J. (1977). Host-guest complexation. 2. Structural units that control association constants between polyethers and tert-butylammonium salts. J. Am. Chem. Soc. 99, 4207–4219. doi: 10.1021/ja00455a001
Tomilov, A. P., Martynov, B. I., and Pavlova, N. A. (2001). Electrochemical alkylation of diethylphosphite. J. Electroanal. Chem. 507, 46–48. doi: 10.1016/S0022-0728(01)00370-9
Wang, T., Sang, S., Liu, L., Qiao, H., Gao, Y., and Zhao, Y. (2014). Experimental and theoretical study on palladium-catalyzed C–P bond formation via direct coupling of triarylbismuths with P(O)–H compounds. J. Org. Chem. 79, 608–617. doi: 10.1021/jo402392t
Witt, D., and Rachon, J. (1996). The direct preparation of the esters of p-nitrobenzylphosphonic acid from p-nitrobenzyl halides. Heteroatom Chem. 7, 359–364.
Xu, J., Zhang, P., Gao, Y., Chen, Y., Tang, G., and Zhao, Y. (2013). Copper-catalyzed P-arylation via direct coupling of diaryliodonium salts with phosphorus nucleophiles at room temperature. J. Org. Chem. 78, 8176–8183. doi: 10.1021/jo4012199
Graphical Abstract
An efficient and convenient method for the synthesis of benzyl phosphonates using KI/K2CO3 as a catalyst and PEG-400 as an environmentally benign solvent has been developed.
Keywords: sustainability, KI +K2CO3, PEG, synthesis of benzyl phosphonates, green chemistry
Citation: Disale S, Kale S, Abraham G, Kahandal S, Sawarkar AN and Gawande MB (2016) A Sustainable and Efficient Synthesis of Benzyl Phosphonates Using PEG/KI Catalytic System. Front. Chem. 4:35. doi: 10.3389/fchem.2016.00035
Received: 12 April 2016; Accepted: 26 July 2016;
Published: 16 August 2016.
Edited by:
Moisés Canle López, University of A Coruña, SpainReviewed by:
Tamer S. Saleh, National Research Centre, EgyptStephen Eustace, Delft University of Technology, Netherlands
Copyright © 2016 Disale, Kale, Abraham, Kahandal, Sawarkar and Gawande. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manoj B. Gawande, mbgawande@yahoo.co.in; manoj.gawande@upol.cz
 Shamrao Disale1
Shamrao Disale1