The Evolution of Equid Monodactyly: A Review Including a New Hypothesis
- 1Department of Ecology and Evolutionary Biology, Brown University, Providence, RI, United States
- 2School of Earth Sciences, University of Bristol, Bristol, United Kingdom
- 3Laboratory of Evolutionary Biology, Department of Anatomy, College of Medicine, Howard University, Washington, DC, United States
The traditional story of horse evolution is well-known: over time, horses became larger, they attained higher-crowned teeth, and they changed from having three toes (tridactyly) to a single toe (monodactyly). Evolution is often perceived as a progression toward some optimum outcome, in this case the “Noble Steed.” However, the evolutionary advantages of monodactyly are not entirely clear, other than the notion that it must somehow be “more efficient,” especially at the larger body size of the genus Equus. It is not commonly appreciated that the reduction of the digits to the monodactyl condition was not the main anatomical foot transition in equid history. Rather, the most important change was the transformation of the original “pad foot” into the more derived “spring foot,” with the acquisition of an unguligrade limb posture, characteristic of the family Equinae. Species within the Equinae tribes—Hipparionini, Protohippini, and Equini—evolved hypsodont teeth and diverged into both small and large body sizes, but monodactyly evolved only within the Equini. Despite the Plio-Pleistocene success of Equus, Hipparionini was by far the richest tribe for most of the Neogene, in terms of taxonomic diversity, numbers of individuals, and biogeographic distribution; but hipparionins remained persistently tridactyl over their duration (17–1 Ma). We propose that the adaptive reasons for monodactyly must be considered in the context of reasons why this morphology never evolved in the Hipparionini. Additionally, Equus inherited monodactyly from smaller species of Equini, and consideration of Miocene taxa such as Pliohippus is critical for any evolutionary hypothesis about the origins of monodactyly. We review the literature on equid locomotor biomechanics and evolution, and propose two novel hypotheses. (1) The foot morphology of derived Equini is primarily an adaptation for increasing locomotor efficiency via elastic energy storage, and the accompanying digit reduction may be circumstantial rather than adaptive. (2) Differences in foraging behavior and locomotor gait selection in Equini during late Miocene climatic change may have been a prime reason for the evolution of monodactyl horses from tridactyl ones.
Introduction
The story of horse evolution is a familiar one, often used as the exemplar for evolutionary patterns (Osborn, 1918; Matthew, 1926; Simpson, 1951), and for this reason frequently maligned by creationists (see Janis, 2007). Although the originally-perceived linear pattern of horse evolution has been reinterpreted as a bushy, branching one (Simpson, 1951; MacFadden, 1992), nevertheless the story of horse evolution remains one of some degree of “progression”: from small animals with many toes and simple, low-crowned teeth (brachydont), to large animals with a single toe and complex, high-crowned teeth (hypsodont). Branches of the family that were not in the direct line of ancestry to the modern Equus have been sidelined in the evolutionary narratives, including the two lineages that migrated from North America to the Old World before the Pleistocene migration of Equus: anchitheriines (large-bodied, specialized browsers) in the early Miocene, and hipparionins (more closely related to Equus, and resembling modern equids in many respects, but persistently tridactyl) in the early late Miocene.
Modern equids, and their more immediate relatives (derived members of the tribe Equini), differ from the great majority of fossil equids in the loss of the medial and lateral digits (digits two and four, the proximal ends of the metapodials are retained as “splint bones”) and the retention of only a single (third) digit; i.e., the condition of monodactyly. Because Equus is the only surviving genus, and it had a great diversity and biogeographic spread of species in the Pleistocene (originating in North America, and reaching South America as well as the Old World, although now extinct in the New World), monodactyly has been perceived as some sort of pinnacle of equid evolution, the ultimate locomotor adaptation (although occasional individuals of modern Equus possess complete additional digits as an atavism, see Figure 1). In addition, because Equus is in general of large body size for the family (modern species ranging between ~200 and 400 kg, some extinct species were as large as ~600 kg), larger than its North American tridactyl relatives, monodactyly has been interpreted as being related to this increased body size. However, Hipparionini in both the New and Old Worlds were a successful and diverse radiation of persistently tridactyl forms, more taxonomically diverse in the late Miocene than the emerging monodactyl Equini lineage, and many Old World hipparionins were as large or larger than modern and fossil members of Equus. (Body mass [BM] estimates in this paper are derived from Shoemaker and Clauset, 2014 [anchitheriines], and Cantalapiedra et al., 2017 [equines]). Thus, body size alone cannot be the reason for the evolution of monodactyly.
Figure 1. A modern example of a polydactyl horse, showing additional digits (in this case, complete second digits) as an atavism. Image from Wikimedia, in the public domain. PSM_V16_D274_ “Outline_of_horse_with_extra_digit_on_each_foot,” Popular Science Monthly vol 16 1879-1880.
Here we review the evolutionary history of equid locomotor morphology, and pose the following question: if monodactyly was such a prominent adaptive feature in the lineage leading to modern equids, then why did it evolve only in this lineage? Why did the equally successful (at least in the Miocene) tridactyl hipparionins not also exhibit any trends toward this “progressive” morphology, especially as some Old World species paralleled species of Equus in hypsodonty and large body size? We propose that monodactyly is not necessarily a “superior” equid adaptation, but that its origins were in changes in foraging behavior in one particular equid lineage in increasingly arid conditions in the late Miocene of North America. Monodactyly in the genus Equus was a fortuitous preadaptation to the greater extent of aridity that affected all higher latitudes in the Plio-Pleistocene. In contrast, Old World hipparionins were well-suited for more wooded conditions with relatively mild seasonality, and their diversity was severely reduced with dramatic climatic shifts at the Mio-Pliocene boundary. We support this hypothesis by weaving together information from equid evolutionary history, foot anatomy, and locomotion, which provide the essential background information that informs our novel proposition.
A Brief History of the Radiation of the Equinae (Family Equidae)
The Equidae consists of three subfamilies: a basal (Eocene) Hyracotheriinae, the late Eocene to latest Miocene Anchitheriinae, and the early Miocene to Recent Equinae. The first two subfamilies are paraphyletic, although the Miocene radiation of large-bodied anchitheres (Anchitheriini) is monophyletic. Equids likely originated in the Old World, and their sister taxon, Palaeotheriidae, was entirely European; but equid Paleogene evolutionary history was largely confined to North America. Both the Anchitheriinae and the Equinae originated in North America, and lineages from both subfamilies migrated to the Old World. The subfamily Equinae is diagnosed by a number of dental characters, including hypsodont cheek teeth with cementum, and a characteristic feature is the “spring foot” (although, as later discussed, this had its origin in derived anchitheriines) (see MacFadden, 1992, 1998). Figure 2 shows a simplified phylogeny of the Equidae, with emphasis on the tribe Equini. Figure 3 shows the distribution of Neogene equids in time and space.
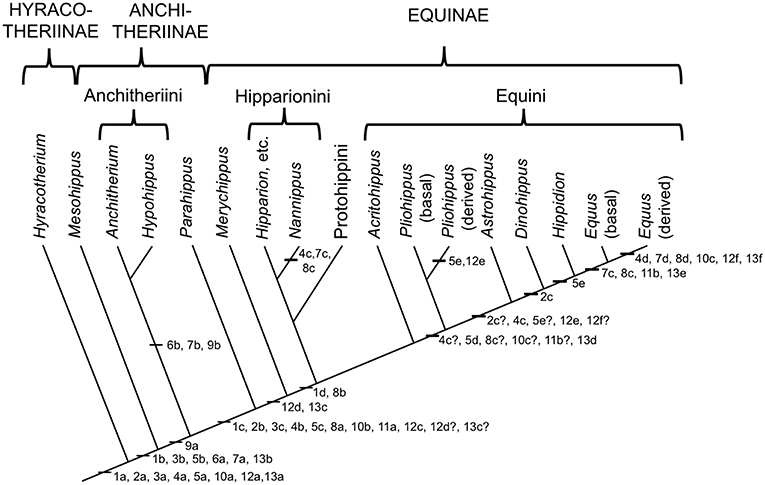
Figure 2. Phylogeny of selected genera (discussed in this paper) within the Equidae based on the phylogeny in (Maguire and Stigall, 2009). Original drawing by CMJ, final figure rendered by James G. Napoli. The numbers on the phylogeny refer to the anatomical characters listed below: note that the “basal” characters are for reference for the generalized condition, and are not necessarily specific to the Equidae. Miohippus (not shown) is crownward of Mesohippus and basal to the Anchitheriini. Characters at the level of Parahippus probably also apply to the other stem equine anchitheres (i.e., crownward of the Anchitheriini) Archaeohippus and Desmatippus (both basal to Parahippus). Anchitherium is an Old World taxon, and North American early Miocene equids originally called Anchitherium should be referred to Kalobatippus (MacFadden, 1992, 1998). Nannippus is singled out because of its unique morphology, and is not the sister taxon to the other hipparionins. Within the Equini, Parapliohippus, Heteropliohippus, and Onohippidium (which may not be distinct from Hippidion) are not shown. 1. Foot posture and foot pad: (a) subunguligrade, foot pad extensive; (b) subunguligrade, foot pad slightly reduced; (c) unguligrade, foot pad converted to digital cushion (3rd phalanges contained within enlarged hoof); (d) Digital cushion reduced slightly. 2. Phalangeal proportions of third (central) digit: (a) 1st phalanx short (length < 2X width), 3rd phalanx longer than 2nd, inclination to ground <26°; (b) 1st phalanx elongated (length > 2X width), 3rd phalanx shorter than second, inclination >26°, <35°; (c) inclination of third phalanx > 40° 3. Third (central) metapodial shaft: (a) similar size to second and fourth metapodials (slightly longer and broader), cross-sectional area oval or elliptical; (b) elongated relative to proximal limb bones, and proportionally longer and broader than second and fourth metapodials; (c) further increased in proportional size, cross-sectional area more circular. 4. Third (central) metapodial distal articulatory surface: (a) narrower in width than metapodial shaft above distal tubercules, sagittal ridge confined to volar surface; articulatory curvature <180°, flexor (volar) surface with greater curvature than extensor (dorsal) surface; (b) sagittal ridge more prominent, encroaches onto dorsal surface, received by groove in first phalanx; articulatory curvature at least 180°, extensor surface with greater curvature than flexor surface, transverse ridge at point of change of curvature (documented for “Merychippus,” presumed to occur in Parahippus); (c) Broader in width than metapodial shaft above distal tubercules, sagittal ridge still more prominent, further encroaching onto dorsal surface; (d) sagittal ridge extremely prominent, fully extended onto dorsal surface. 5. Other metapodials and associated digits: (a) complete metapodial 5 and digit 5 retained in manus, metapodial 1 and digit 1 lost; metapodials 2 and 4 large, not bound to central metapodial, associated digits likely contacted ground during regular locomotion; (b) metapodial 5 and digit 5 greatly reduced or lost, metapodials 2 and 4 more tightly bound to central metapodial; (c) shafts of metapodials 2 and 4 reduced, tightly bound to central metapodial by ligaments, associated digits smaller, no longer contact the ground during regular locomotion, but may do so during more extreme performance; (d) sagittal ridge/groove for articulation of 1st phalanges on metapodials 2 and 4 now very faint or lost entirely; shafts of metapodials 2 and 4 further reduced in width, associated digits further reduced in size; (e) shafts of metapodial 2 and 4 confined to proximal two thirds of central metapodial (i.e., transformed to “splint” bones), associated digits lost. 6. Scars for cruciate sesamoid ligaments (proximal volar surface of 1st phalanx): (a) present; (b) enlarged. 7. Scar for central sesamoidean ligament (mid volar surface of 1st phalanx of 3rd digit): (a) present; (b) enlarged; (c) reduced; (d) lost/merged with V-scar for oblique ligaments. 8. Scars for oblique sesamoid ligaments (proximal to mid volar surface of 1st phalanx of 3rd digit): (a) present, small, round, extend no more than 30% down phalanx; (b) enlarged, forming incipient V-scar, extend further down phalanx (<50% of bone); (c) elongated, extend >50% down phalanx; (d) merge to form more distinct V-scar, extend 66% down phalanx. 9. Scars for straight sesamoid ligament (proximal volar surface of 2nd phalanx of 3rd digit): (a) present; (b) enlarged. 10. Suspensory ligament: (a) probably fully muscular (= interosseous muscle of 3rd metapodial and digit); (b) at least partially tendinous; (c) fully tendinous (evidenced by loss of volar gulley on third metapodials). 11. Size of proximal sesamoid bones of 3rd digit: (a) increase in size to support the suspensory apparatus; (b) further enlarged. 12. Tarsus: (a) astragalus articulates predominantly with navicular, distal articulation rounded, ridges of trochlea directed anteriolaterally (angle with sagittal plane 14–20°), considerable intertarsal movement possible, relatively long astragalar neck; (b) astragalar neck shorter; (c) more restrictive tibia–astragalar articulation, distal articulation of astragulus with navicular wider and flatter; (d) ridges of astragalar trochleae higher, restricting motion to parasagittal plane, intertarsal movement more restricted by ligaments; (e) width of astragalus greater than length; (f) ridges of astragalar trochlea narrower and directed more anteriorly (angle with sagittal plane 10–15°), reduced lateral swing of foot, little or no intratarsal movement possible. 13. Knee joint: (a) angle at knee joint ~102°, large and rugose area on tibia for attachment of semitendinosus muscle for limb retraction–reflects more flexed knee joint and tibial rotation on limb retraction; (b) angle at knee joint ~108°; (c) angle at knee joint ~131°; (d) area of attachment of semitendinosus of moderate size; (e) angle at knee joint ~140°; (f) Area of attachment of semitendinosus weak, angle at knee joint ~150°.
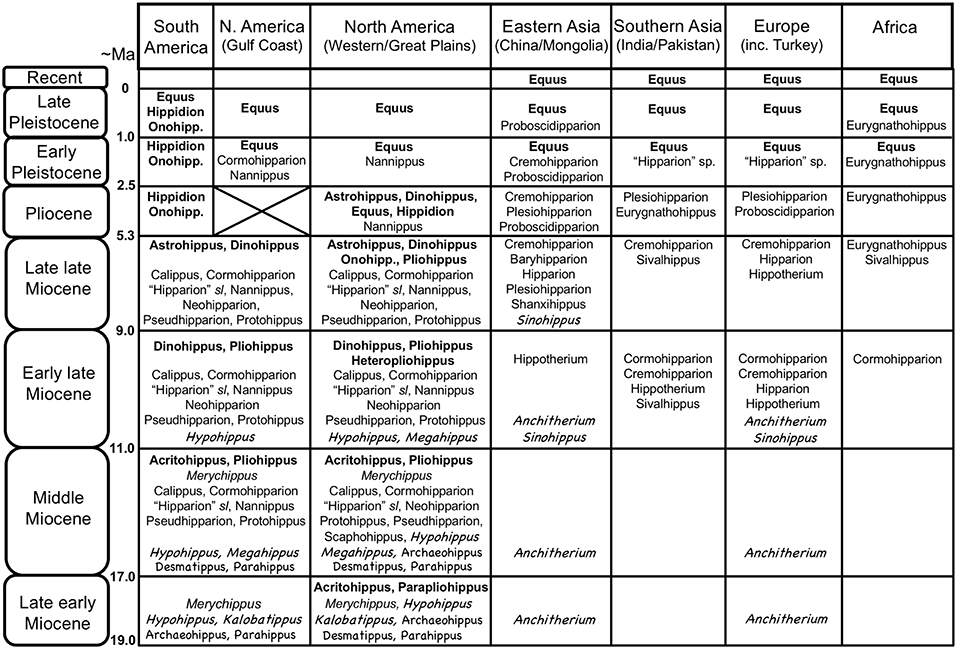
Figure 3. Global distribution of Neogene equid genera, showing the diversity of the different families/subfamilies. Original drawing by CMJ, final figure rendered by James G. Napoli. Key to equid taxonomy: Roman type = Equinae: bold face = Equini (Onohipp. = Onohippidium); regular type = Hipparionini (including Protohippini) (sl = sensu lato); italics = “Merychippus” sensu stricto (i.e., basal species, more derived species not shown, but none persist past the middle Miocene). Chalkboard type = Anchitheriinae: regular type = stem Equinae anchitheres; italics = Anchitheriini. Although anchitheriine equids are only considered briefly in this paper, their distribution in time and space is interesting, and their persistence alongside of the Miocene Equinae is often disregarded. Information from Janis et al. (2008), MacFadden (2013), Salesa et al. (2004), the NOW database, Fossilworks, and Bernor et al. (1996). Divisions within epochs are approximate to accommodate both North American Land Mammal Ages and Eurasian MN/MQ zones + Land Mammal Ages. Late Pleistocene = Rancholabrean/MQ2; early Pleistocene = Irvingtonian + late Blancan/MQ1; Pliocene = early Blancan (+ very latest Hemphillian)/MN14-16/Ruscinian (no faunas known from the North American Gulf Coast); late Miocene = Hemphillian/MN13-11/Turolian + late Vallesian; early late Miocene = Clarendonian/MN9-10/early Vallesian + late Astracean; middle Miocene = Barstovian/earliest Clarendonian/MN5-8/Astracean + late Orleanian; late early Miocene = Hemingfordian/MN3(later part)-4/middle Orleanian.
The Equinae is subdivided into the tribes Hipparionini (late early Miocene to Pleistocene), Protohippini (middle Miocene to latest Miocene) and Equini (late early Miocene to Recent). Members of these tribes will be referred to as “hipparionins,” “protohippins,” and “equins,” respectively, while “equines” refers to members of the subfamily Equinae.
The taxon Merychippus was originally considered the basal type of equine (e.g., Simpson, 1951), but species ascribed to this genus actually represent a paraphyletic grade of smaller, less hypsodont Equinae, and the taxon name is now usually placed in quotes. “Merychippus” gunteri is the most basal equine, “Merychippus” primus is the sister taxon to the grouping of the Equinae tribes, and other “Merychippus” species mostly belong to the Hipparionini (including the type species “Merychippus” insignis: Hulbert and MacFadden, 1991): note that “Merychippus” insignis is the sister taxon of Cormohipparion goorisi, which is at the base of the North American Cormohipparion/Old World hipparionin radiation (Woodburne, 2007). The Equinae tribes are mainly diagnosed by dental characters, but while most of the Equinae retained the tridactyly seen in basal “Merychippus” species, monodactyly evolved only within the Equini. Full monodactyly (complete loss of medial and lateral digits) was first apparent in the early late Miocene (Clarendonian) in derived species of Pliohippus. All Clarendonian and younger Equini species were likely monodactyl although, as detailed below, this is not a simple issue.
The North American hipparionins comprise the genera “Hipparion” (not the same taxon as the Old World genus of that name), Neohipparion, Pseudhipparion, Nannippus, and Cormohipparion (MacFadden, 1992, 1998). Old World hipparionins take their origin from North American Cormohipparion, with their oldest occurrence being in the Pannonian Basin Zone C between 11.4 and 11.0 Ma (Bernor et al., 2017). There was an extensive Old World radiation including the genera Hipparion sensu stricto, Hippotherium, Cremohipparion, Sivalhippus, Eurygnathohippus, Plesiohipparion, Proboscidipparion and Shanxihippus (Bernor et al., 1996, 2018; Bernor and Sun, 2015).
The “core” (i.e., the monodactyl genera) of the Equini comprises the Pliocene to Recent genus Equus; the Plio-Pleistocene predominantly South American genera Hippidion and Onohippidium (Hippidion is shown by mitochondrial DNA to be the likely sister taxon to Equus; Der Sarkission et al., 2015); and, as successive outgroups to this grouping, Dinohippus (paraphyletic with respect to Equus), Astrohippus, Heteropliohippus, and Pliohippus. The basal equins (all tridactyl) are now considered to be Acritohippus (= “Merychippus” tertius, “M.” stylodontus, and “M.” isonesus) and (crownward) Parapliohippus (= “Merychippus” carrizoensis) (see Kelly, 1995, 1998). With the exception of Equus, Hippidion, and Onohippidium, the Equini were all exclusively North American.
The North American genera Protohippus, Calippus, and Scaphohippus (= “Merychippus” sumani and “M.” intermontanus) were originally placed within the Equini, basal to the above-mentioned Equini taxa (see e.g., Hulbert and MacFadden, 1991; MacFadden, 1992, 1998). More recent analyses have assigned them to their own tribe, Protohippini, as the sister taxon to the Hipparionini (e.g., Kelly, 1995, 1998; Pagnac, 2006), and we include them with the hipparionins in our discussions here.
Hipparionins (including protohippinins) and equins differed in their craniodental morphology, and probably had different feeding ecologies. Hipparionins had molariform teeth that had more complex occlusal enamel morphology than those of the equins, often with densely plicated enamel, and the occlusal surface relief was usually higher [but note that the highly hypsodont Eurygnathohippus woldegabrieli (Bernor et al., 2013) had very low occlusal relief], and their teeth were usually less hypsodont than those of equins (Famoso et al., 2016). In addition, hipparionin mesowear tends to be indicative of browsing-to-mixed feeding while that of the equins is usually more indicative of grazing (Mihlbachler et al., 2011, but note that dental microwear studies do not support this conclusion; e.g., Bernor et al., 2013; Semprebon et al., 2016).
The radiation of the North American Equinae was explosive in the late early Miocene (late Hemingfordian, ca. 17.5 Ma), and reached a peak in diversity in the late middle Miocene (late Barstovian, ca. 14 Ma) through the early late Miocene (Clarendonian, ca. 11.5–8.5 Ma) (see Figure 3). During this time, individual localities could have as many as eight contemporaneous equid species [data from Janis et al. (2004a)], and the hipparionins were more diverse than the equins. By the latest Miocene (Hemphillian) the equid radiation was declining, reflected in the overall number of taxa, the abundance of individuals in the fossil record, and the number of sympatric species at fossil localities (three to four species, down to one to two species in the Pliocene; Janis et al., 2004a). The genus Equus is first known from the earliest Pliocene. Only a few of the Miocene taxa survived into the Pliocene: out of the Equini, Dinohippus, Astrohippus, Onohippidium and Hippidion and (the latter taxon surviving until the end of the Pleistocene in South America); out of the Hipparionini, Cormohipparion and Nannippus (the latter taxon possibly surviving into the early Pleistocene) (see Janis et al., 2008). The Pleistocene of North America was the time of extensive radiation of species of Equus, which migrated to the Old World at 2.6 Mya and is recognized initially by Eurasian and African “stenonine” horses (Azzaroli, 2000; Wang and Deng, 2011; Alberdi and Palombo, 2013; Bernor et al., 2018).
Cormohipparion was the founding genus for Old World hipparions, although it was not the earliest known form, which was Hippotherium, known from ca. 11.2 Ma in Europe and China (Bernor et al., 2017, 2018). Cormohipparion has been recorded from 10.8 Ma levels of Pakistan and Turkey (Bernor et al., 2003), and slightly younger (10.5 Ma) from Algeria. Sivalhippus likewise diverged in the Vallesian, ca. 10.5 Ma, in Indo-Pakistan (Wolf et al., 2013) and underwent a local radiation there, then extended its range into China and East Africa. Eurygnathohippus is known from late Miocene aged deposits of Kenya, Ethiopia, Libya and Morocco (Bernor et al., 2012). Plesiohipparion originated in China, and was later known from Turkey, India, and Spain. Proboscidipparion originated in the latest Miocene of China, and in the Plio-Pleistocene was known from China and Turkey (Bernor et al., 2018; Sun et al., 2018). In contrast to the North American Miocene, only two or three species of equids are known in any given faunal locality in the Old World Miocene.
Equus was first known in the Old World in Eurasia at 2.6 Ma (the Equus Datum, Lindsay et al., 1980; Azzaroli, 2000; Bernor et al., 2018), and diverged into a dozen or more species during the Pleistocene (not including the seven surviving species). The genus first appeared in Africa at around 2.3 Ma. Distribution of extant Old World clades of Equus has been reviewed by Bernor et al. (2010).
The Evolution of Unguligrady and Monodactyly in Equids
Structure and Function of the Modern Equid Foot
Extant equids have a single main digit (the third) and retain only the proximal shafts of the medial (second) and lateral (fourth) metapodials (also known as the “splint bones”), which extend approximately halfway down the central metapodial. Although these structures are often described as “vestigial,” their proximal ends clearly have an indispensable function in supporting the articulation of the manus and pes with the carpus and tarsus, respectively. The monodactyl litoptern (extinct South American ungulate) Thoatherium is often described as “more advanced” than equids, as it has apparently lost almost all of the shaft of the medial and lateral metapodials (MacFadden, 1992). But these conditions of monodactyly are convergent: there is no reason to think that the retention of the metapodial shafts in Equus represents some intermediate stage in the evolution of monodactyly. The anatomy of the equid foot, and the major tendons and ligaments is shown in Figure 4. Anatomical descriptions below are taken from Nickel et al. (1986), Stashak (2006).
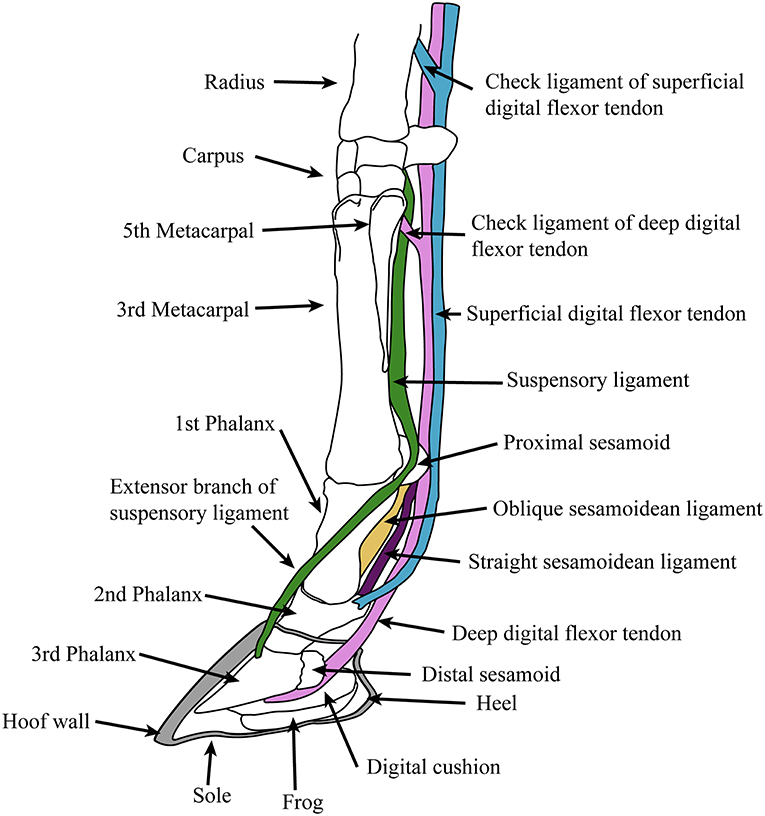
Figure 4. Diagram of supportive tendons and ligaments of the equine foot (only selected ligaments, referred to in the text, are shown). The hoof (covering the third phalanx and the distal portion of the second phalanx) is shown in cross section. Original drawing by CMJ, final illustration rendered by James G. Napoli.
The equid foot posture is unguligrade: unguligrady is seen elsewhere today only among ruminant artiodactyls, where the foot is formed of two separate digits in the “cloven-hoof” condition. In equids, weight is directed through the tip of the third (distal) phalanx, which is encased in a keratinous hoof and supported on the volar (= plantar or palmar) side by a fibrous digital cushion. The ventral surface of the foot is composed of keratinous epithelial structures; posteriorly by a thick V-shaped structure termed the “frog,” which provides traction and aids the digital cushion in its shock-absorbing function, and anteriorly by a thinner sole.
The principal joint in the foot is the fetlock, between the metapodial and the first (proximal) phalanx (there is little motion at the interphalangeal joints). This joint is held at an angle of around 25° to the vertical at rest, but is capable of extending (= dorsi-flexion) to an angle of 90° during extreme performance (galloping or jumping: intermediate angles of 40°-60° are observed during more routine locomotion) (McGuigan and Wilson, 2003). This fetlock flexion is the basis of the equid “spring foot,” which both reduces the impact of the forces acting on the foot during rapid locomotion and, by means of stretching the flexor tendons, acts to store elastic energy in these tendons, saving up to 40% of locomotor energy during foot recovery (Biewener, 1998). The elongation of the first phalanx is an important component of this mechanism, allowing for the fetlock to be held higher off the ground, resulting in greater stretch of the flexor tendons. However, this increase in distance between foot and fetlock magnifies the moment arm of the ground reaction force around the fetlock joint, necessitating a firm ligamentous apparatus supported by stout proximal sesamoid bones (Thomason, 1986). Note that this “spring foot” action is essentially passive: similar force vs. length curves are seen in feet of living horses and in applying force to the limbs of cadavers (McGuigan and Wilson, 2003).
The “spring foot” involves a complex series of supportive ligaments. The suspensory ligament, derived from the interosseous muscle of the third metapodial and digit, holds the fetlock in a sling, limiting both flexion and hyperextension. It originates from the proximal volar surface of the third metapodial and the distal carpal (or tarsal) bones, and runs along the volar surface of the central metapodial. Near the distal end of the metapodial it splits into two slips, inserting onto the proximal sesamoid bones on either side of the volar metapodial surface. Two extensor branches then extend forward to join with the common digital extensor tendon, which inserts on the extensor process on the proximal dorsal aspect of the third phalanx.
Sesamoidean ligaments bind the proximal sesamoids to each other and to the foot bones, and transmit forces experienced during locomotion to the suspensory ligament (Thomason, 1986). These include the cruciate ligaments, the lateral (oblique) ligaments and the straight (rectus) ligament. These ligaments leave scars on the volar surface of the phalanges, and so their history through equid evolution can be traced (see legend for Figure 2). Other supportive foot ligaments include various collateral ligaments that run laterally from one foot bone to the neighboring one, and three annular ligaments that encircle the volar side of the foot, superficial to all of the other ligaments and tendons; the most proximal of these, the palmar annular ligament, is of particular importance in binding the proximal sesamoid bones and associated ligaments to the rest of the foot.
The primary elastic energy storage is in the digital flexor tendons, both deep and superficial, which are stretched during locomotion with the extension of the fetlock. These structures are muscular only in their most proximal portions, small super-pinnate muscles that function as spring dampeners (Wilson et al., 2001). The flexor tendons course superficial to the suspensory and sesamoidean ligaments and have “check ligaments” that aid in the passive suspensory apparatus of the foot (see Figure 4); these prevent excessive stretch of the tendons, and may act as additional parallel elastic elements (Wilson et al., 2001). The superficial digital flexor tendon divides into two before inserting onto the second phalanx on either side of the straight sesamoidean ligament. The deep digital flexor tendon, which experiences the greatest locomotor strains and contributes the most to elastic energy savings (Biewener, 1998), passes over the distal sesamoid (articulating with the proximal third phalanx and distal second phalanx) and inserts onto the flexor cortex of the third phalanx.
Locomotion in Modern Horses
The familiar gaits of horses, from slow to fast, are the walk, trot, and gallop (a slow gallop is called a canter). In all tetrapods there is a fundamental difference between the walk gait and other, faster gaits (collectively termed “running”). In walking, the animal vaults over strut-like limbs, and the center of mass (COM) is at its highest during the foot supportive phase: the mechanics are basically those of an inverted pendulum, with exchange of potential and kinetic energy contributing to the recovery of up to 70% of mechanical energy. In running, the animal engages in spring-mass mechanics on compliant legs, and the COM is at its lowest during foot support. Up to 40% of mechanical energy can be recovered via storage and release of elastic energy in tendons and ligaments: this results in a greater efficiency of locomotion (less total energy expenditure per unit distance traveled).
Walking gaits do not usually involve a period of suspension (i.e., an aerial phase, when all four feet are off the ground), while running gaits usually do, but this is not invariant. With increasing speed within any one gait, the limbs are subjected to greater vertical forces (and hence to more bone stress) as the limbs move faster, and the amount of time each foot is on the ground decreases: these musculoskeletal stresses may be the trigger to switch gaits (see Biknevicius and Reilly, 2006; Biknevicius et al., 2006, for review). In horses, at least, while the walk involves pendular mechanics, and the trot spring-mass mechanics, the gallop is considered to involve some combination of the two. The energy savings from pendular mechanics in walking are 25–47%, while those in the gallop are 7–14%: in contrast, in trotting elastic energy recovery savings are 21–45% (greater in larger horses) (Reilly and Biknevicius, 2007). Horses are unusual among the mammals studied in that their running gaits are no cheaper than their walking gait, and running may be more expensive than walking in larger horses. This is likely due to extremely efficient walking in horses, rather than any deficiency with running, and horses may store some elastic energy during their walk gait (fetlock extension is observed in walking horses), thereby reducing locomotor costs (see Reilly and Biknevicius, 2007).
The walk and the trot are both symmetrical gaits. During walking, a four beat gait, each foot is moved in sequence (i.e., single-foot) in an ipsilateral pattern (i.e., the right hind is followed by the right fore, etc.) with no aerial phase in the stride cycle. During trotting, a two beat gait, contralateral pairs of legs are moved together, with two aerial phases in the stride cycle, the horse essentially bouncing from one diagonal pair of supportive legs to the other. The gallop is an asymmetrical, four beat gait, with the sequential support of the two hind limbs being followed by the two forelimbs; there is a “gathered” aerial phase before the hind limbs are again engaged in support (see Clayton, 2004, for a description of horse gaits). There is little contribution from spinal flexion to the stride in the gallop (except at the lumbo-sacral junction), although some elastic energy may be stored and recovered in vertebral column ligaments (Alexander, 1988). In contrast to the horse “stiff-back” mode of galloping, cursorial carnivorans such as the cheetah (and also some smaller artiodactyls) practice a “flexible-back” gallop, where there are two aerial phases and considerable spinal flexion, which potentially contributes to elastic energy storage as well as to stride length (the “transverse gallop” vs. “rotary gallop” of Bertram and Gutmann, 2009).
A few types of domestic horses practice a gait called the running-walk or amble, best known as the tölt of Icelandic horses. This gait is practiced at similar speeds to the trot (~11–18 km/h), and is often used in place of a trot in the walk-trot-gallop sequence, although the running-walk may extend into the speeds where trotting horses will switch to a gallop (up to 30 km/h) (Clayton, 2004; Barrey, 2013). As many as 16 varieties of this gait and other “single-foot” gaits have been recorded among different horse breeds, collectively referred to as “gaited horses” (Nicodemus and Clayton, 2003), and the propensity for gaited locomotion has been traced to a single mutation in domestic horses (both the mutation and the gait are unknown in other equids) (Promerová et al., 2014). However, while the running-walk is rare in extant equids, trackways of the Pliocene hipparionin from Laetoli, Tanzania show that it was using this gait (Renders, 1984), a point to which we shall return.
The running-walk has the same lateral sequence single-foot pattern as the regular walk, usually lacking an aerial phase, but is speeded up so that up to three feet may be off the ground at any one time. Despite this similarity of footfall sequence to the walk, the tölt has the spring-mass mechanical properties of a running gait: the limb stiffness is comparable to that of trotting horses, but the vertical motion of the COM is much less. Icelandic horses employ the tölt over a similar range of speeds as the trot, and may extend this gait past the usual trot-to-gallop transition speed (Biknevicius et al., 2006).
Evolution of the Modern Equid Condition
Figure 2 shows the acquisition of the various derived features of the equid foot in the form of characters on a simplified phylogeny. This information is derived from Camp and Smith (1942) and Sondaar (1968), both writing about the forelimb, and Hussain (1975) on the hindlimb. Note that there are conflicts with some taxonomic nomenclature, as discussed in the previous section, especially with species of “Merychippus.” Even though museum numbers are usually provided for the specimens examined, it would not be easy to determine their correct taxonomic affinities.
With these caveats in place, some obvious trends can be noted (the numbers refer to the numbers on the phylogeny). The largest cluster of changes is with the appearance of the “spring foot” anatomy (#1c), heralding the loss of the foot pad and the transition to an unguligrade foot posture. This involves changes in digit proportions (#2b,3c,5c), the medial and lateral digits no longer contacting the ground during regular locomotion; changes in the articulation at the fetlock joint that limit lateral motion but allow for greater parasagittal rotation (#4b); changes in phalangeal proportions, with an elongated first phalanx, and a more vertically-positioned third phalanx that is now shorter than the second phalanx (#2b); and associated postural changes in the hind limb (#12c,12d,13c). There is also evidence of the development of the ligamentous suspensory apparatus with the appearance of scars for the oblique sesamoidean ligaments (#8a), and the suspensory ligament was now probably at least mostly tendinous (#10b) (see Figure 4).
The “spring foot” is often thought to be a novel feature of the Equinae (e.g., Thomason, 1985, 1986), but its inception was actually at the level of the stem equine anchitheres (here represented by Parahippus) (see also O'Sullivan, 2008, on the lengthening of the first phalanx being the key indicator of this postural change). Although more basal anchitheres such as Mesohippus and Miohippus retained a pad foot, there were a number of changes between hyracotheres and Mesohippus that relate to a greater complexity of supportive ligaments as well as changes in the relative sizes of the pedal bones. Note that the acquisition of the straight sesamoidean ligament (#9a) in equids more derived than Mesohippus and Miohippus may be related to support of a greater body weight than in earlier equids (i.e., > ~50 kg), and that the very large-bodied (>200 kg) anchitheres Megahippus and Hypohippus (Anchitheriini) possess a number of ligamentous features indicative of restricted motion at the fetlock (#6b,7b,8b). A number of other morphological changes are seen with the transition to the “spring foot” that also relate to classic anatomical “cursorial adaptations,” such as the great reduction or loss of the distal ulna and fibula (MacFadden, 1992). The proximal sesamoid bones, involved in the support of the ligamentous suspensory apparatus, may also become larger at the transition to the “spring foot” (#11a; Mesohippus [F:AM 74048] has smaller proximal sesamoids than Parahippus [MCZ 17877]).
Following the acquisition of the “spring foot,” there is little functional change observed in the pedal morphology of most tridactyl Equinae, although Camp and Smith (1942) proposed a reduction in the size of the digital cushion above the level of “Merychippus” (#1d), and the scars for the oblique sesamoidean ligaments now extend to around 50% of the volar surface of the first phalanx (#8b), forming the beginnings of the V-scar (see Figure 5) that is most prominent in modern species of Equus. There is little or no discussion in the literature of any instance of reduction of medial and lateral digits in North American hipparionins (except possibly for Nannippus, see below), although Deng et al. (2012) report a reduction in the size of these digits in the long-limbed Hipparion (=Plesiohipparion) zandaense from the Pliocene of Tibet. Deng et al. (2012) also report other morphological features that resemble those of monodactyl equids: a larger medial trochlear ridge on the distal femur, longer and more distinct V-scars on the first phalanx, a relatively reduced width across the distal metapodial tuberosities, and a better developed sagittal ridge on the distal articular surface of the third metapodial. Parker et al. (2018) employ a “toe reduction index” in their examination of equid morphological traits, and their Figure 3 suggests that there has been toe reduction in several hipparionin lineages, although not in any systematic phylogenetic fashion. We consider that the possibility of digit reduction in North American hipparionins requires a more systematic investigation. There is no report of medial and lateral digital reduction in Old World hipparionins other than that by Deng et al. (2012).
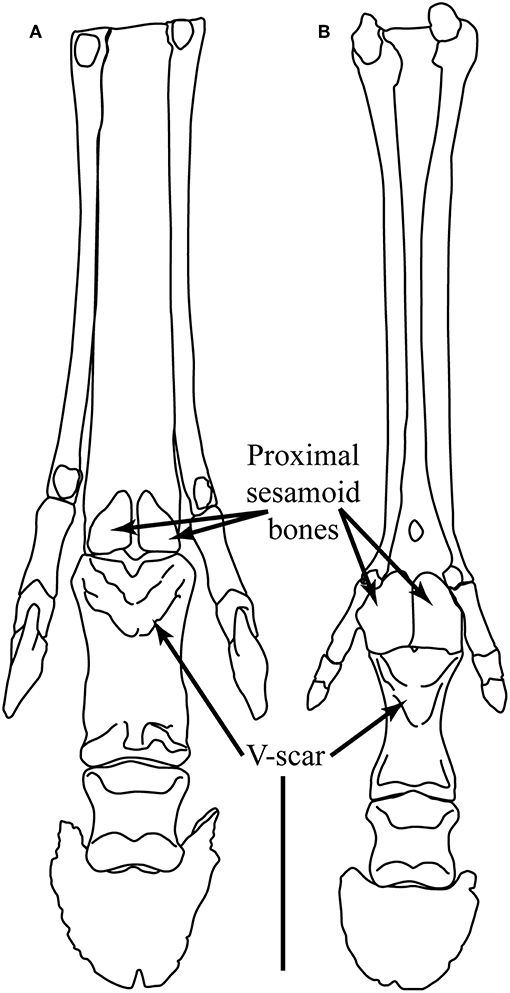
Figure 5. Plantar view of the left foot of (A) Hippotherium primigenium and (B) Pliohippus pernix. Hippotherium is from the late Miocene of Höwenegg (Germany), and the illustration is modified from Figures 6.23.2 and 6.31.1 in Bernor et al. (1997). Pliohippus is based on F:AM 60803 from the early late Miocene (early Clarendonian) of Nebraska (June Quarry, Burge Member of the Valentine Formation) (photographs taken by CMJ, third phalanx mirror-imaged). Scale bar = 5 cm. Illustration by James G. Napoli.
Camp and Smith (1942), Sondaar (1968), and Hussain (1975) all note that the small (dwarfed) hipparionin Nannippus (BM~50–100 kg) paralleled the monodactyl equins in aspects of the fetlock joint articulation and suspension (#4c,7c,8c). Sondaar (1968) claims that the medial and lateral digits were reduced in size, but Parker et al. (2018) report a toe reduction index less than most other North American hipparionins, and similar to Parahippus. Sondaar (1968) also notes a number of features of the articulation for carpal and tarsal bones on the central metapodials that show convergence with the condition in Equus. Additionally, Nannippus also appears to have had a stiffer lumbar region than other hipparionins, approaching the condition in Equus (Jones, 2016).
The evolution of monodactyly in the Pliohippus–Equus portion of the Equini phylogeny involves many other changes in locomotor anatomy. [Note that the species of Pliohippus considered by Camp and Smith (1942) and Sondaar (1968) have been reassigned to Dinohippus; some information on Pliohippus presented here is derived from personal observations of CMJ, but the precise morphology of species within this taxon needs further investigation, as noted by the question marks on the characters in Figure 2]. While a couple of features occurred convergently between derived (monodactyl) Pliohippus and the other monodactyl equids (not only the complete loss of digits two and four [#8a], but also the relative broadening of the astragalus [#12e]), there are a number of other features shared by this clade of Equini that indicate a strengthening of the ligamentous suspensory apparatus, increased rotation of the fetlock joint, and the greater restriction of hind limb motion to the parasagittal plane.
Anatomical changes shared by the clade of Pliohippus–Equus include the following (note that some of these are queried at this level and require further investigation): the increasing encroachment of the distal sagittal ridge on metapodials to the dorsal surface of the articulation (#4c,d); the loss of the metapodial volar gully indicating a completely tendinous suspensory ligament (#10c); enlarged proximal sesamoid bones indicative of an enhanced suspensory apparatus (#11b); the elongation and fusion of the oblique sesamoid ligaments to form a distinct V-scar (#8c); and a somewhat less-flexed knee joint (#13d). Changes occurring in more derived Equini (crownward of Pliohippus) include a more vertical position of the third phalanx within the hoof (#2c); a further less-flexed knee joint (#13,e,f); and an astragalus that limits the motion of the hind limb to the parasagittal plane to a greater extent (#12e,f). Note that a number of the features seen in extant Equus are present in a less derived state in more basal species of the genus, as well as in other monodactyl equids.
In summary: (1) The acquisition of the “spring foot” was a key feature for the evolution and radiation of the Equinae, allowing for increased efficiency of locomotion: but, as noted by Thomason (1986), it may also be a liability, as it magnifies the forces acting on the musculoskeletal system. (2) Monodactyl Equinae differ from tridactyl ones not only in the loss of the medial and lateral digits, but also in having an enhanced “spring foot,” with evidence of greater extension at the fetlock joint (and stronger ligaments to support this motion), accompanied by limb movement more restricted to the parasagittal plane.
The Origin of Monodactyly
Monodactyly is known in derived species in the genus Pliohippus and all Equini genera crownward of Pliohippus (see Figure 2). Pliohippus is first known in the United States from the late Barstovian (late middle Miocene, 12.6–14.8 Ma, although it has been reported from the early Barstovian of Mexico; see Janis et al., 2008). Pliohippus survived until the end of the Miocene, but was only common in the mid-Miocene (Barstovian to early Clarendonian) (MacFadden, 1998). Pliohippus was originally considered to be directly ancestral to later monodactyl equids (e.g., Stirton, 1940), but this genus has a number of its own unique features, including complex facial fossae that are smaller or absent in crownward Equini (MacFadden, 1998), and so was excluded from ancestry of more derived equins. However, all recent equid phylogenies place Pliohippus as the sister taxon to the other monodactyl members of the Equini. There has been considerable confusion between species of Pliohippus and the more derived Equini genera Astrohippus and Dinohippus (see MacFadden, 1998). Dinohippus is an early late Miocene to Pliocene genus (Clarendonian to early Blancan), and Astrohippus is a latest Miocene to Pliocene genus (Hemphillian to early Blancan); both genera are distinct from Pliohippus in the reduction of the preorbital facial fossae (to a greater extent in Dinohippus than in Astrohippus). Dinohippus (BM~170–500 kg) also tends to be of larger body size than Pliohippus (BM~110–170 kg) or Astrohippus (BM~125–190 kg).
Pliohippus has also been noted to be variable in the incidence of monodactyly, with tridactyl individuals reported. The most well-known example is among individuals of a single death assemblage of Pliohippus pernix from the Ashfall Fossil Beds in Nebraska (early Clarendonian, Cl-2 ca. 11 Ma) (Voorhies, 1994; Tucker et al., 2014), although the limb morphology of these fossils has not been studied in a quantitative fashion. Hussain (1975) notes incidences of both monodactyly and tridactyly among the specimens of Pliohippus in the Frick collection at the American Museum of Natural History (New York) (AMNH). He claims that the first monodactyl Pliohippus is F:AM 60812, known from Bear Creek Quarry in the Cap Rock Member of the Ash Hollow Formation (the same member and formation as the Ashfall Fossil Beds). Based on information in MacFadden (1998) this is also likely to be Pliohippus pernix. Another specimen of Pliohippus pernix studied by Hussain (1975), F:AM 60803, is slightly older (from June Quarry, Burge Member of the Valentine Formation, earliest Clarendonian, Cl-1, ca. 13 Ma), and is quite definitely tridactyl, even though the medial and lateral digits are small (see Figure 5). We know of no systematic study of variability of digit number in species of Pliohippus in general. Figure 5 compares the forefeet of the Old World hipparionin Hippotherium primigenium, and a tridactyl specimen of Pliohippus pernix. Note the smaller size of the medial and lateral digits in Pliohippus, and also the larger proximal sesamoid bones and the greater extent of the incipient V-scar.
Because Pliohippus includes tridactyl individuals it has been common to claim that monodactyly evolved convergently in Pliohippus and Equus (e.g., Azzaroli, 1992, who claimed that monodactyly evolved on four separate occasions, additionally in Astrohippus, and Hippidion + Onohippidium). However, as noted above, tridactyl individuals of Pliohippus have phalanges of the medial and lateral digits that are reduced in size in comparison to other tridactyl equines, and they have a number of other pedal features elsewhere seen only in monodactyl equids. We consider that functional monodactyly evolved only once within the Equidae, in the common ancestor of Pliohippus and the more derived members of the Equini. However, the complete loss of the medial and lateral digits (i.e., the transition to full monodactyly) evidently occurred convergently at least twice, as discussed below.
Although the phylogeny in Figure 2 places monodactyly as a feature of all equids crownwards of Pliohippus, it is not known for certain whether or not any species or individuals of Astrohippus or Dinohippus retained medial and lateral digits. MacFadden (1998) claimed that both genera are monodactyl, as far as is known. Although MacFadden (1992, p. 255) originally noted that Dinohippus was variably tridactyl, this is actually a reference to the Ashfall specimens of Pliohippus, referred to as Dinohippus in Voorhies (1981). This is also the source of the claim in Parker et al. (2018) that Dinohippus was variably tridactyl (pers. comm. of CMJ with Abigail Parker). Azzaroli (1992) also appeared to have been under a similar misunderstanding in claiming for variability in digit reduction in Protohippus: again, he was referring to the Ashfall Pliohippus.
An examination by CMJ of a collection of metapodials of Astrohippus from Guymon area in Texas (late Hemphillian) in the AMNH revealed no evidence of scars for the medial and lateral metapodials extending further than around halfway down the bone. Hussain (1975, p. 218) claimed that Dinohippus “also became monodactyl at about the same time” (as Pliohippus), but he did not further elaborate on any incidence of tridactyly in Dinohippus. However, there is some evidence for the retention of side toes in Dinohippus. A UNSM specimen (27855) definitively referred to as Dinohippus evidences lateral and medial first phalanges. An AMNH specimen referred to as Dinohippus sp. (F:AM 116128), from the Burge Member of the Valentine Formation (the same member as Pliohippus pernix, which would make it younger than any other recorded member of the genus), evidences complete (but very slender) medial and lateral metapodials with articulatory facets on their distal ends. From its age, it seems more likely that this individual would represent a specimen of Pliohippus, but the well-preserved skull (of similar size to P. pernix) shows only a shallow dorsal preorbital facial fossa, and no evidence of a malar fossa, resembling the condition in Dinohippus.
Further research is clearly needed to clarify the issue of the exact pattern of the origin of monodactyly in the Equini: that is, to determine whether there were only two instances of convergence in the loss of the medial and lateral digits (in Pliohippus and in the common ancestor of the more derived Equini), or if there were one or more instances within genera in the Equini lineage crownward of Pliohippus (i.e., whether basal members of Astrohippus and/or Dinohippus were fully monodactyl).
Hypotheses for the Evolution of Monodactyly
Most hypotheses about equid monodactyly relate to the notion of some sort of “improved” form of locomotion, accompanying increasing body size. Almost all authors perceive the acquisition of the “spring foot” as some sort of “precursor” to monodactyly, which of course it is in absolute terms, but it is not irrevocably linked with this further change in locomotor anatomy. Nevertheless, the reasons for evolving the “spring foot” should first be considered.
The Evolution of the “Spring Foot”
Thomason (1986) considered increased body size (i.e., larger than Mesohippus) to be an important factor. Mesohippus species were 40–60 kg in body mass, and the “break point” between more basal anchitheriines and the stem-equine anchitheriines appears to be around 70 kg. Larger animals experience greater change in the rate of momentum during locomotor foot impact, and the shock-absorption capacity of the “spring foot” would compensate for this. Additionally, recovery of elastic energy is also relatively less in smaller animals. However, size alone cannot be the reason as the “spring foot” never evolved in the Anchitheriinae, some of which attained sizes as large as any extant Equus and, as noted earlier, acquired their own ways of stabilizing the fetlock joint. Thomason (1986) also cited the likely complicating factors of habitat choice and ground compliance, noting that “spring-footed” equids were open-habitat animals (and a harder ground surface would mean greater foot concussion on impact). This observation may be extended to the “spring-footed” stem equine anchitheriines, which commenced their radiation in the late Oligocene, before the spread of grasslands in the early Miocene of North America, but at a time when open habitats were likely prevalent (see Damuth and Janis, 2011). Open-habitat ungulates have larger home ranges and travel greater daily distances (Janis and Wilhelm, 1993), and the energy-saving potential of the “spring foot” may have become important at this time.
Although this change to an unguligrade posture is termed a “cursorial adaptation,” and is usually assumed to be adaptive for fast running, Reilly and Biknevicius (2007) consider that it may have been more related to slow transportation (walking) over long distances, especially as in modern wild horses trotting and galloping only comprises around 2.5% of their locomotor repertoire (A similar point about cursorial anatomy and efficiency for walking was made by Janis et al., 2004b).
Reasons for Retaining the Medial and Lateral Digits
Thomason (1985, 1986) also considered the transition to monodactyly, noting that most authors are more concerned about the function of the medial and lateral digits in the tridactyl equines than the reason for their loss. The consensus appears to be that the side toes would not contact the ground during locomotion, but might prevent sprains due to hyperextension of the central digit (Simpson, 1951), and/or offer additional support during extreme fetlock extension, such as during slipping on soft ground (Renders, 1984). Sondaar (1968) considered the retention of the side toes to be adaptive for locomotion on soft or muddy ground. Thomason (1986) cited Gromova (1952) for the notion that the medial and lateral digits may have acted to prevent lateral dislocation of the central digit, a function assumed in Equus by the extension of the sagittal ridge onto the dorsal surface of the distal metapodial articulation. Note, however, that the prominence of this ridge is a feature of derived Equus species, and the extent to which the ridge extended onto the dorsal articular surface in Pliohippus is not well-established. If this ridge was performing an essential function that was lost along with the medial and lateral digits, it seems probable that a modern Equus-like condition would be acquired sooner in monodactyl equids.
Thomason (1986) also cited Zhegallo (1978), who considered that the medial and lateral digits could help absorb the forces of impact through the ligamentous connections to the central digit. However, while this may explain the reason for the retention of medial and lateral metapodials, it does not address the reason for retention of the medial and lateral digits. This hypothesis may, in fact, explain the retention of much of the shaft of the medial and lateral metapodials in monodactyl equids.
Shotwell (1961) presented evidence to show that, during the early late Miocene (Clarendonian) of North America, hipparionin horses were found in more mesic, woodland-savanna-like environments, many of which (for example, those in the Northern Great Basin) did not contain monodactyl equines or their ancestors. But in the latest Miocene North American (Hemphillian) hipparionins declined and species of monodactyl equins spread in association with an encroachment of more arid, steppe-like habitats. Old World hipparionins also underwent a precipitous decline in diversity at the end of the Miocene, but were not replaced by equins; rather, a reduced number of hipparionin genera persisted between 5.3 and 2.6 Ma; Equus first occurred in the Old World 2.6 Ma. Shotwell (1961) considered that the retention of the side toes would have rendered tridactyl equines more agile, providing better ground traction, and made them better at dodging predators in the woodland-savanna environments, while in the more open grassland habitats preferred by Pliohippus, where high speed locomotion would have been required for predator escape. Shotwell (1961) does not speculate as to why a monodactyl foot would be better for high speed, except to say that in open environments “agility was then a burden.” Bernor et al. (1997), in their description of the Höwenegg Hippotherium primigenium assemblage, argued that the slender bauplan, flexible spine and tridactyl foot were adapted to leaping and springing in their woodland setting.
Researchers have reexamined the issue of habitat differences between tridactyl and monodactyl horses in the past decade, availing themselves of modeling techniques and large databases on fossil occurrences unavailable to Shotwell. Maguire and Stigall (2008) employed a phylogenetic biogeographic analysis of the Equinae, and showed some regional differences between the tribes, both in the areas of their original diversification and in their subsequent dispersal. In terms of differences between tridactyl and monodactyl equines, they note that protohippins largely diversified in the Gulf Coast areas, while hipparionins and equins initially inhabited the same northern and western biogeographic regions. But with increasing late Neogene aridity and the spread of more open grasslands, by the Pliocene the hipparionins became restricted to the more mesic Gulf Coast region, while equins (primarily the genus Equus) diversified elsewhere. Maguire and Stigall (2008) followed Shotwell (1961) in suggesting that this difference was related to foot anatomy, tridactyl equids faring better in “muddy substrates.”
Maguire and Stigall (2009) used niche modeling techniques, determining different habitat types within the Great Plains biogeographic region to examine the distribution of species occurrences. They concluded that a major difference between the mid Miocene interval (Barstovian and Clarendonian) and the later Miocene and Pliocene interval (Hemphillian and Blancan) was for habitats to become less fragmented, with a more homogenous type of grassland habitat predominating. Species ranges became broader, possibly resulting in reduced rates of speciation that contributed to the late Neogene overall taxonomic decline of equids in general. However, Parker et al. (2018), also used niche modeling techniques (employing ecomorphological traits such as body size, hypsodonty and toe number), and showed no evidence of differences in habitat occupancy between monodactyl and tridactyl equids, and no specific correlation of any of these traits with grassland habitats. They attributed the evolution of monodactyly to increasing body size and “other selective pressures” rather than to habitat choice, and they followed the general trend in considering tridactyly adaptive for “wet substrates.” The latter hypothesis is rejected based on the Old World distribution of hipparionins and their ecological context (Bernor et al., 1996, 2010; Eronen et al., 2009; Figure 3 herein).
In summary, it appears that, while hipparionins and equins may have occupied different biogeographic ranges to some extent, and that equins expanded in cooler and more arid regions in the late Neogene while hipparionins retreated from them, the fact that both types of equids had similar habitat occupancies for much of the later Miocene indicates that habitat preference per se cannot be the reason for the different locomotor morphologies. We propose later that, rather than different habitat occupancy, monodactyl and tridactyl horses had differences in their preferred gaits and economical travel speeds.
Reasons for Losing the Medial and Lateral Digits
There are two main hypotheses relating to digit loss: the notion of “inertial load,” and the notion of “beam strength.” Both were summarized by Thomason (1986). However, both hypotheses depend on the assumption that Equus has a larger body mass than all tridactyl equines, and that body size increase played an important role in this locomotor transition.
The “inertial load” hypothesis is perhaps the most common one, with the consideration that the additional toes add weight to the distal portion of the limb, and so would increase the metabolic cost of locomotion. This extra weight could be tolerated at smaller body sizes, especially if the medial and lateral digits retained some function; but with increasing body size the “inertial load” would become relatively greater, and there would be selection for digit reduction and loss. This hypothesis falters not only because many species of Old World hipparionins were within (or even exceeded) the size range of extant species of Equus (see e.g., Cantalapiedra et al., 2017), but also because the species of Pliohippus (whether monodactyl or tridactyl) were of similar body size to the contemporaneous (late Miocene) tridactyl equids, with body masses in the 100–200 kg range (excluding smaller “dwarfed” forms such as species of Calippus and Nannippus). A further consideration is that, because the central digit becomes enlarged in monodactyl forms (especially apparent in Equus; this would require investigation for the other monodactyl taxa), the larger central digit may equal the mass of the original three digits (in the derived tridactyl condition), and the overall inertia of the foot may not actually be reduced.
The “beam strength” hypothesis starts with the observation that a single beam is stronger in resistance to bending than two (or more) beams made of the same quantity of material and of similar cross-sectional shape (Alexander, 1980; Biewener, 1998). Thomason (1986) expanded on this idea, proposing a combination of single beam strength and reduction of inertia as the reason for the loss of the medial and lateral digits. However, it seems to us that while the “single beam” concept could explain a transition from a Mesohippus-like condition, with three digits of subequal size, to an Equus-like single digit condition, this transition was more-or-less accomplished with the enlargement of the central digit with the evolution of the “spring foot” in derived anchitheres. This notion of “beam strength” does not seem appropriate for a consideration of reasons for monodactyly in the Equinae, where all forms have a central digit that is much larger than the medial and lateral ones. Note that the cross-sectional diameter of the central metapodial scales with positive allometry in equids (Thomason, 1986), in part reflecting the “spring foot” transition when three subequal digits were essentially replaced by a single, larger one. Simple beam resistance does not explain the persistence of small medial and lateral digits in most tridactyl equines, nor their subsequent loss.
McHorse et al. (2017) undertook an elegant study on the beam strength and likelihood of fracture failure of equid metapodials, examining not only the strength of the central digit but also the contributions of the medial and lateral digits. They investigated how these biomechanical functions changed with increasing body size and digit reduction, quantifying the latter by employing a “toe reduction index” (TRI). They studied 11 different equid genera ranging from Hyracotherium to Equus (most represented by two specimens), and also included two specimens of tapir (Tapirus bairdii). Pliohippus was represented by a single specimen of P. pernix from Ashfall (University of Nebraska State Museum [UNSM] 52297), presumably one of the tridactyl individuals as its recorded TRI is barely less than the included hipparionins. The results they presented are for the metacarpals, but results for the metatarsals were similar.
The resistance of the central metacarpal to bending stress (i.e., beam strength) was much greater in Equus than in any tridactyl equine. Tapirus had similar resistance to the tridactyl equines, probably due to its large size, while the values were considerably less in the other equids (anchitheres and hyracotheres). The maximum stresses experienced by the central metacarpal during loadings for “normal locomotion” (trotting) were within the zone of bone safety factors for all taxa, but the results were different for “performance locomotion” (galloping or jumping). Here the stresses exceeded the safety factor range in most equids, the exception being Equus and three of the tridactyl equines (including Pliohippus); for most equids (and the tapirs), stresses in the central metacarpal were only kept below dangerous levels (approaching or exceeding fracture stresses) if contribution from the medial and lateral digits was taken into account.
McHorse et al. (2017) summarized their results by noting that both metacarpal cross-sectional area and second moment of area (= resistance to bending) increase with positive allometry in equids, a trend unusual among mammals, but most of the tridactyl equids (all considerably smaller than extant Equus) would have experienced unsafe levels of stress in performance locomotion without contributions from the medial and lateral digits. They noted that the increasing size of the central digit compensates for the increasing body mass throughout equid evolution, and the medial and lateral digits may have been lost simply because of relaxed selection once the central digit was strong enough to counter all locomotor forces (as apparent in most of the tridactyl Equinae); but the “inertial load” of the retained side toes may have reduced speed or increased locomotor costs, and so they would ultimately be selected against.
The research of McHorse et al. (2017) greatly increases our knowledge about the evolutionary biomechanics of the equid limbs, but their final conclusion is that their results support the hypothesis that “body mass was a potential driver of digit reduction.” However, they did not consider data from Equus-size Old World hipparionins: for example, Eurygnathohippus hasumensis (BM~500 kg; see Bernor et al., 2010) and Proboscidipparion sinense (BM~465; see Sefve, 1927; Qiu et al., 1987; Bernor and Sun, 2015; Bernor and Sen, 2017) are both fully tridactyl, and thus body size increase cannot be the major driver of monodactyly. We again note that digit reduction begins at the level of Pliohippus, equins no larger than the contemporaneous hipparionins.
An Alternative Hypothesis for the Evolution of Monodactyly
Anatomical Features That Accompany Monodactyly
Monodactyly is accompanied by a suite of anatomical features related to the foot suspensory apparatus, many of which are apparent even in tridactyl individuals of Pliohippus. While it might be assumed that these pedal modifications compensate for the lack of the supportive function of the medial and lateral digits, it seems to us more likely that this notion should be reversed: that is, that the enhanced suspensory apparatus rendered the medial and lateral digits redundant. With this perspective, reasons for an enhanced suspensory apparatus should be the primary consideration.
The equid suspensory apparatus limits over-extension of the fetlock and helps to “spring” the foot back from an extended position. The amount of extension possible at the fetlock joint relates to the degree of curvature of the distal articular surface of the metapodials, as also reflected by the dorsal extent of the sagittal ridge around that surface. This anatomy (highly curved articular surface with dorsally-extended sagittal ridge) distinguishes Equus from “Merychippus,” indicating a greater extent of fetlock extension (dorsi-flexion) in Equus (Thomason, 1985, 1986). Thomason (1986) estimates a maximum extension angle of around 65° (to the vertical) in “Merychippus,” in contrast to 90° in Equus. Although this anatomy has not been systematically examined in other members of the Equinae, it appears that a trend toward the Equus condition (not even fully realized in early species of the genus) accompanies the trend toward monodactyly (see Figure 2). A more developed suspensory apparatus allows for a greater extension at the fetlock, and hence for a greater degree of stretching of the flexor tendons. This in turn allows for greater amount of elastic energy recovery during locomotion, and hence a greater efficiency of locomotion. Thus, we propose that the transition to monodactyly is correlated with selection for greater efficiency of locomotion, irrespective of any considerations of the loss of the “inertial load” of the side toes.
Another anatomical feature apparently correlated with monodactyly is the change from a more flexible back to a stiffer one, with restriction of sagittal movements of the lumbar region. Jones (2016) examined the lumbar region of a series of North American fossil horses and concluded that anterior and middle lumbar flexibility decreases throughout equid evolution (although posterior lumbar flexibility remains approximately the same). She showed shifts of decreasing flexibility to occur at the transition between hyracotheriines and basal anchitheriines (e.g., Mesohippus), between basal anchitheriines and stem equine anchitheriines (e.g., Parahippus: i.e., at the level of the acquisition of the “spring foot”), and then to an Equus-like condition at the level of Hippidion (BM≥250 kg). She proposed that hyracotheres may have practiced a “flexible-back” type of gallop, but that more recent equids were more restricted. As previously mentioned, Nannippus (the only hipparionin included) shows more restricted lumbar flexibility, converging on the condition seen in Equus.
Jones (2016) concluded that increasing body size was the main driver in the evolution of equid lumbar stability. But, again, size cannot be the only factor: not only did the Old World hipparionins remain persistently tridactyl, they also had a lumbar region that evidences a much greater degree of mobility than Equus. Hippotherium primigenium, a basal Old World hipparionin from Höwenegg (late Miocene of Germany), was of a similar size (BM~295 kg) to a common zebra (Equus quagga burchelli), but also evidenced a capacity for greater medio-lateral lumbar rotation (Bernor et al., 1997). Hippotherium had comparably weakly-developed zygapophyses and expansion of the articular surfaces of the transverse processes; thoracic and lumbar vertebrae with long spinous processes indicative of well-developed epaxial musculature; and a sacrum that was long and narrow in comparison with Equus, with a more narrow flare of the ala ossis sacri that form the sacroiliac joint, and longer spinous processes with expanded distal tubercles (see Figure 6). Bernor et al. (1997) interpreted this spinal anatomy as enabling Hippotherium to leap and spring and to make sharp turns while running, advantageous in its original woodland habitat.
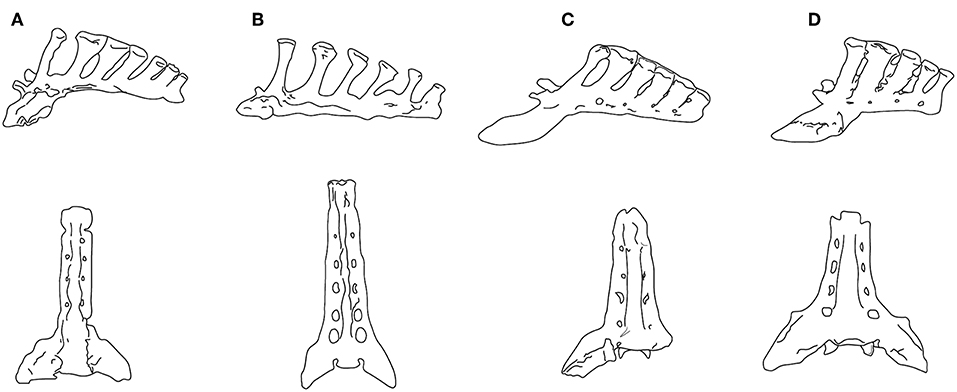
Figure 6. Sacra in left lateral view (upper) and ventral view (lower). (A) Neohipparion coloradense (F:AM 69511); (B) Hippotherium primigenium, modified from Figure 5.4.2 in Bernor et al. (1997); (C) Pliohippus pernix (F:AM 60872); (D) Equus grevyi (AMNH 82036). (F:AM = Frick Collection, American Museum of Natural History). All specimens drawn to the same size, not to scale. Photographs taken by CMJ. Illustration by James G. Napoli.
Although Jones (2016) interpreted the lumbar flexion of Pliohippus pernix (BM~175 kg) to be similar to that of the tridactyl equids Parahippus and “Merychippus,” its sacrum is more like that of Equus. While the sacrum of the contemporaneous (and similarly-sized) hipparionin Neohipparion resembles that of Hippotherium, the one of Pliohippus is both shorter and broader, with spinous processes that are shorter, more posteriorly inclined, and more appressed to each other, approaching the Equus condition (see Figure 6). The hipparionin Pseudhipparion (UNSM 27837) has a sacrum of similar anatomy to Neohipparion. The hipparionin sacral anatomy appears to be the more basal condition: similarly long sacra can be seen on mounted skeletons in the Fossil Mammal Gallery at the American Museum of Natural History, including the anchitheriines Parahippus and Hypohippus, and the basal equin Acritohippus (= “Merychippus”) quinni, while Dinohippus has a shorter, more Equus-like sacrum. We interpret this morphology as further indication of a more flexible back in hipparionins than in the monodactyl equins, with a stiffer back and shorter sacrum being a derived condition in the equins.
A Hypothesis of a Change in Preferred Gaits
We have shown that monodactyl equines combine a more extensive suspensory apparatus in the foot with a less flexible back than hipparioninins, and propose that this combination of anatomical features is indicative not only of selection for increased locomotor efficiency (via elastic return of some proportion of the energy lost over a stride cycle) but also of a difference in the preferred gait for travel at speeds faster than a walk. The trackways of a hipparionin from the Pliocene age locality of Laetoli, Tanzania show it to have been performing a running-walk (Renders, 1984). Of course, a single trackway is insufficient to determine that all tridactyl horses habitually used this gait: but we discuss below why the anatomy of monodactyl equines might be specifically adapted to the use of a trot for economical locomotion over distances, and why a running-walk may have been the preferred intermediate-speed gait for tridactyl equines. This proposal of differences in gait selection depends, in part, on the hypothesis (discussed in the following section) that, during their initial appearance in the late Neogene of North America, monodactyl equines adopted a diet that required a greater extent of daily or seasonal roaming behavior than that of the contemporaneous tridactyl forms.
Although, as previously discussed, the mechanical properties of the trot and running-walk gaits are similar, the trot is likely to be a more efficient gait for distance travel, while the running-walk might have advantages if economy over distance was not an issue. Biknevicius et al. (2006) note that peak ground reaction forces of individual limbs are smaller in tölting horses than in trotting horses (see also Waldern et al., 2015), which may mean that a running-walk could be comfortably sustained at speeds at which a trotting horse would have transitioned to a gallop. They also speculate that, with at least one foot being on the ground at all stages of the stride cycle, the running-walk might provide a both a better base of support and enhanced proprioception, and hence afford superior performance over uneven surfaces. The notion of enhanced proprioception is especially interesting, as this may have been a functional reason for retaining non-weight-bearing medial and lateral digits.
However, a disadvantage of the running-walk is that it may be metabolically more expensive than trotting. In a comparison of the two gaits practiced by the same Icelandic horses, Waldern et al. (2015) noted that these horses had a shorter stride and a higher stride frequency when tölting than during trotting, and that they also experienced less limb compression. As the metabolic energy expended per stride is a constant (Taylor et al., 1982), this implies that a running-walk is more expensive than trotting, and Waldern et al. (2015) noted that the metabolic power required was around 5% greater. They also noted (citing Ingolfsdottir, 2013) that riders observe that Icelandic horses prefer the trot to the tölt when fatigued. In horses, metabolic costs increase less steeply with increasing speed in trotting than in either walking or galloping (Minetti et al., 1999), which contributes to it being an efficient gait, but it seems unlikely that this would be true for the running-walk.
As previously noted, monodactyl horses have anatomical evidence of a greater maximum extent of fetlock extension (dorsi-flexion) than tridactyl ones. Waldern et al. (2015) note a lesser degree of limb compression in tölting vs. trotting horses: this implies that the running-walk relies less on elastic energy return than the trot, as limb compression occurs primarily via hyperextension of the fetlock joint (especially in the forelimb). The corollary of this is that the trotting gait in horses is more reliant on fetlock hyperextension and elastic energy recovery than the running-walk. Interestingly, Biewener (1998) noted that the maximum energy storage (40%) occurred during the transition from walk to trot, and that energy storage in the trot is greater than in the gallop. Biewener (1998) also noted that it is the deep digital flexor tendons that experience the greatest strains and contribute most to elastic energy savings.
How might the relative stiffness of the back (including the shorter and broader sacrum) in monodactyl horses be correlated with the above observations? The trot gait in extant equids is a stiff-backed gait compared with the gallop, which entails a greater extent of lumbar flexion (Harris, 2016). However, perhaps it is not that the stiffer back aids a trotting gait, but more that it is a consequence of having more “springy” legs. More competent leg springs might reduce the need for the demands for elastic function in the back during the gallop: stiffening the back might then be advantageous in reducing epaxial muscle activation required for stiffening the torso during locomotion (we thank Jim Usherwood for this proposition). This hypothesis could be explored by comparing the size of the vertebral spinous processes (supporting the epaxial musculature) in hipparionins and equins: note that these are large in Hippotherium (Bernor et al., 1997), and that the sacral spinous processes are larger in the hipparionins depicted in Figure 6. It is also interesting in this regard that among gaited domestic horse breeds, those practicing a running-walk are favored by longer backs and hips (i.e., the sacral area).
In summary, it appears that the running-walk might have certain locomotor advantages, especially on uneven surfaces or mixed terrestrial substrates such as found in wooded and forested settings, but a trot gait would be more metabolically efficient for distance travel at a medium-speed sustained gait. A tridactyl horse employing a trot would benefit from increased elastic energy storage (more so than when doing a running-walk), and so there would be selection for an increased extent of fetlock hyperextension and concomitant increased support for this motion from the ligamentous suspensory apparatus. Thus, we propose that an initial change in behavior (change in preferred gait, due to a greater extent of daily roaming) would result in selection for change in morphology (e.g., Lister, 2014); even small changes would incrementally reduce locomotor costs.
It is evident from the morphology that monodactyl horses are maximizing their capacity for elastic energy recovery, but why would this also be associated with the loss of the medial and lateral digits? It is conceivable that there was no active selection for the loss of the medial and lateral digits, but rather that this was a corollary of enlarging the central digit. In equid ontogeny, the foot originally develops with five digits and all but the central one are reduced by means of cell death (Cooper et al., 2014). In tridactyl equids there must have been a lesser extent of apoptotic digit reduction; but could it be possible that selection for a more predominant third digit might have the side effect of a greater reduction of the second and fourth ones during ontogeny? In particular, given the importance of the digital flexor tendon in elastic energy storage, perhaps an initial part of this selection was for a larger distal sesamoid bone for its support and insertion. This notion is admittedly speculative; but our point is that the essence of pedal anatomy in monodactyl equids may primarily be a large central digit in a foot with greater ligamentous support and an enhanced suspensory apparatus. The loss of the medial and lateral digits may not necessarily be adaptive per se, but, rather, may merely represent an ontological consequence.
Summary and Conclusions
Monodactyly is often perceived as an evolutionary “improvement” in equid locomotion. But this notion of “improvement” does not explain the persistence of tridactyl horses for millions of years after monodactyly first appeared, nor why only a single equid lineage made the transition to (greater) reduction and/or loss of the medial and lateral digits. The Old World hipparionins were highly diverse, and paralleled the New World equins in changes in body size (both increases and decreases) and hypsodonty (Cantalapiedra et al., 2017), yet they showed no trend toward monodactyly. MacFadden (1992) notes that, in the overall scenario of late Neogene equid evolution, it was the tridactyl horses that were the most successful radiation.
Monodactyly (or incipient monodactyly) was first apparent in North America, with the genus Pliohippus in the middle Miocene around 15 Ma (definitively monodactyl forms apparent by ca. 10 Ma). At this time tridactyl equines were the predominant forms (~10 genera, see Figure 3). Tridactyl equines did not decrease in diversity until around 5 Ma (by which time they had become increasingly restricted to more southern regions). Tridactyl equines became extinct in North America at ca. 2 Ma, and at <1 Ma in southern Asia and Africa, by which time the only remaining monodactyl equin was the genus Equus (see MacFadden, 1992, 1998; Janis et al., 2008). Considering the early evolution of monodactyly, rather than the eventual success of Equus, monodactyl horses must initially have been doing something different in a world where tridactyl horses were predominant.
We propose that the divergence of the lineage of equines that became monodactyl from their tridactyl relatives had its origins in a difference in diet and foraging behavior. Although niche modeling has demonstrated that North American Miocene monodactyl and tridactyl horses did not live in completely different habitats (Parker et al., 2018), nevertheless there must have been differential selection on the locomotor morphologies of tridactyl and monodactyl lineages. We propose that North American Miocene monodactyl horses adopted a feeding regime that entailed a greater extent of roaming behavior, on a daily and/or seasonal basis. African zebras evolved as part of a migratory ungulate community that was dependent on long-distance roaming. This original (Miocene) diet may have comprised a greater percentage of grass than in the tridactyl equines, as implied by some (but not all) studies of dental morphology and wear; but our hypothesis does not depend on the actual composition of the diet, only of the foraging behavior necessary to subsist on it.
A greater extent of roaming behavior would have led to selection for limb morphology that supported more efficient distance transport. This might simply have been for more efficient walking, as proposed by Reilly and Biknevicius (2007). Zebras usually walk while migrating (Pennycuick, 1975), but the locomotory issue for the members of the equin lineage may have been for a combination of speed and efficiency for daily travel between patchy resources of food. Wild horses are known to trot for daily distance travel; the trot was used for efficient transport by the US cavalry, and endurance equestrians habitually select the trot gait (Harris, 2016; Egenes, 2017). The trot gait involves a much greater degree of storage of elastic energy in the flexor tendons than the walk: as we propose that it is this aspect of locomotor performance (i.e., greater efficiency resulting from enhanced elastic energy storage) that drove the evolution of monodactyly, it is a plausible proposition that a preference for a trot gait at speeds between walk and gallop was the selective factor for anatomical change. The adoption of the trot as the preferred medium-speed gait may have been a key difference between equins and hipparionins, given that trackways evidence a running-walk in a hipparionin (Renders, 1984). This proposed locomotor difference may also explain the difference in the lumbar and sacral regions of hipparionins (longer and more flexible) and equins (shorter and stiffer), and also the less-flexed knee joint of equins (Hussain, 1975). In modern horses, as previously noted, the running-walk gait may have advantages for stability and proprioception over uneven surfaces, but it is metabolically more demanding than a trot.
Although both running-walk and trot gaits in modern horses rely on spring-mass mechanics with compliant legs, there is a greater extent of limb compression in trotting horses, and thus likely a greater amount of return of stored elastic energy in the foot tendons and ligaments. Both monodactyl and tridactyl equines had a “spring foot” that would have promoted storage and recovery of elastic energy during locomotion; but monodactyl equines show osteological evidence for greater amount of hyperextension of the fetlock (morphology of the distal metapodial articular surface) and a more extensive suspensory apparatus to support the distal foot during this hyperextension (larger proximal sesamoid bones). The foot anatomy of monodactyl equines primarily reflects a greater extent of energy storage and recovery during locomotion, and hence more economical transport: the loss of the medial and lateral digits may not be adaptive per se, but may simply be a consequence for selection for a foot that is superior in economy for distance transport.
If monodactyl horses were initially in the minority, how did monodactyly, the distinguishing feature of the genus Equus, come to predominate and eventually be the only form of equid foot anatomy? Monodactyly in North American equids first appears in the later Miocene, when higher latitude cooling and aridification was starting to dominate Cenozoic climatic regimes (Zachos et al., 2001). However, aridification had an earlier onset in North America than in the Old World (late Miocene vs. Plio-Pleistocene); this may explain the success of many mammals migrating to the Old World in the Plio-Pleistocene (Equus among them), as they would have been preadapted to the emerging Old World arid conditions (Eronen et al., 2012). Thus, the late Miocene North American equids would have been experiencing increasingly arid conditions, while the Old World ones were still inhabiting a more mesic world. This may explain why no Old World tridactyl lineage adopted the type of roaming behavior that we hypothesize characterized the emerging New World monodactyl lineage.
Note that with further increasing aridity in North America in the earliest Pliocene, the remaining tridactyl equines became more or less restricted to more southern regions, while the monodactyl equines diversified in the more northern grasslands. Maguire and Stigall (2008) attributed this biogeographic divergence to substrate differences, but we consider it more likely that the different equine lineages were following divergent dietary preferences and, hence, divergent foraging behaviors. It is also of interest that the one hipparionin that did adopt some Equus-like locomotor morphology of the feet and back, Nannippus, was the only hipparionin to survive into the Pliocene in the more northern regions of North America (see Figure 3). Note also that this was a dwarfed form (estimated BM of Pliocene N. peninsulatus = 75 kg), which contrasts with the hypotheses for the evolution of Equus pedal morphology being related to an increase in body size (and also note that the monodactyl litoptern, Thoatherium, was even smaller, ~25 kg.).
When the genus Equus migrated to the Old World at the start of the Pleistocene (2.6 Ma) the hipparionins were still present, although of reduced diversity; but hipparionins persisted alongside of Equus until sometime later than 1.0 Ma in China and Africa. Equus may have had the advantage in the Old World Pleistocene, as with the encroaching aridity and cooling it would have been preadapted for more efficient distance transport. Would Old World hipparionins have evolved monodactyly if not for the presence of Equus? The question is unanswerable, but it is worth noting that the lineage leading to Equus had at least 10 million prior years of evolutionary history leading to the foot anatomy seen in modern species of the genus. As previously noted, although monodactyly was established somewhere around the Astrohippus/Dinohippus stage in the latest Miocene, even early species of Equus lacked the full suite of derived anatomical features seen in extant equids (see Figure 2). It may well be that the Old World hipparionins would never have “progressed” to monodactyly, as was the fate of their New World relatives and, moreover, they never showed this tendency in their 10 Ma (or more) of evolution in Eurasia and Africa.
We conclude that the monodactyly of Equus represents an exaptation for the cooler and more arid world of the Plio-Pleistocene and the present day. We propose that the origins of monodactyly were in the divergent foraging behaviors of late Miocene North American hipparionin and equin lineages, leading to selection in the latter lineage for a greater efficiency of locomotion. This novel locomotor adaptation, manifested in the monodactyl condition, set the stage for the genus Equus to survive and flourish globally in the Plio-Pleistocene, while the tridactyl horses that had dominated the late Miocene were not so fortunate. In terms of monodactyly, at least, Equus was fundamentally a lucky genus in the grand scheme of equid evolution.
Author Contributions
CJ conceived of the idea, and provided the information on North American equids. RB provided the information on Old World equids. Both authors contributed to the writing of the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor is currently organizing a Research Topic with one of the authors, RB, and confirms the absence of any other collaboration.
Acknowledgments
We thank Judy Galkin and Jin Meng at the American Museum of Natural History (New York) for access to the collections of fossil horses, to Sara Ketelsen for access to the recent horses at this same institution, and to Russ Secord for access to the collections of fossil horses at the University of Nebraska State Museum. Thanks also to Jim Usherwood (Royal Veterinary College, London) for extensive help with, and comments on, the sections on equine locomotion; Jon Baskin and Kelly Lessel for help with references; Brianna McHorse for reviewing our summary of her research and providing photographs of Parahippus (Brianna is coincidentally also writing a review on the evolution of equid monodactyly); John Damuth, and reviewers Spencer Lucas and David Marjanović for comments on the manuscript; and James G. Napoli for the illustrations. CJ visits to North American museum collections have been supported by the Bushnell Foundation (Brown University). RB research on fossil equids has been supported by past NSF grants EAR0125009, 1113175, 1138908, and 1558586.
References
Alberdi, M., and Palombo, M. R. (2013). The late early to early middle pleistocene stenonoid horses from Italy. Quatern. Int. 288, 25–44. doi: 10.1016/j.quaint.2011.12.005
Alexander, R. M. (1980). “Terrestrial locomotion,” in Mechanics and Energetics of Animal Locomotion, eds R. M. Alexander and G. Goldspink (London:Chapman and Hall), 168–203.
Alexander, R. M. (1988). Elastic Mechanisms in Animal Movement. Cambridge: Cambridge University Press.
Azzaroli, A. (1992). Ascent and decline of monodactyl equids: a case for prehistoric overkill. Ann. Zool. Fennici 28, 151–163.
Azzaroli, A. (2000). On Equus livenzovensis Baigusheva 1978 and the “stenonid” lineage of equids. Palaeontograph. Ital. 87, 1–17.
Barrey, E. (2013). “Gaits and interlimb coordination,” in Equine Locomotion, eds W. Back and H. M. Clayton (New York, NY: Elsevier), 85–97.
Bernor, R. L., Armour-Chelu, M., Gilbert, H., Kaiser, T. M., and Schulz, E. (2010). “Equidae,” in Cenozoic Mammals of Africa, eds L. Werdelin and W. L. Sanders (Berkeley, CA: University of California Press), 685–721.
Bernor, R. L., Boaz, N., and Rook, L. (2012). Eurygnathohippus feibeli (Perissodactyla, Mammalia) from the late Miocene of Sahabi (Libya) and its evolutionary and biogeographic significance. B. Soc. Paleontol. Ital. 51, 39–48.
Bernor, R. L., Gilbert, H., Semprebon, G., Simpson, S., and Semaw, S. (2013). Eurygnathohippus woldegabrieli sp. nov. (Perissodactyla: Mammalia) from the Middle Pliocene of Aramis, Ethiopia (4.4 Ma.). J. Vertebr. Paleontol. 33, 1472–1485. doi: 10.1080/02724634.2013.829741
Bernor, R. L., Göhlich, U. B., Harzhauser, M., and Semprebon, G. M. (2017). The Pannonian C hipparions from the Vienna Basin. Palaegeogr. Palaeoclim. Palaeoecol. 476, 28–41. doi: 10.1016/j.palaeo.2017.03.026
Bernor, R. L., Koufos, G. D., Woodburne, M. O., and Fortelius, M. (1996). “The evolutionary history and biochronology of European and Southwest Asian Late Miocene and Pliocene hipparionine horses,” in The Evolution of Western Eurasian Later Neogene Faunas, eds R. L. Bernor, V. Fahlbusch, and H. W. Mittmann (New York, NY: Columbia University Press), 307–338.
Bernor, R. L., Scott, R. S., Fortelius, M., Kappelman, J., and Sen, S. (2003). “Systematics and evolution of the Late Miocene Hipparions from Sinap, Turkey,” in The Geology and Paleontology of the Miocene Sinap Formation, Turkey, eds M. Fortelius, J. K. S. Sen, and R. L. Bernor (New York, NY: Columbia University Press), 220–281.
Bernor, R. L., and Sen, S. (2017). The early pliocene Plesiohipparion and Proboscidipparion (Equidae, Hipparionini) from Çalta, Turkey (Ruscinian Age, c. 4.0 Ma). Geodiversitas 39, 28–41. doi: 10.5252/g2017n2a7
Bernor, R. L., and Sun, B. (2015). Morphology through ontogeny of Chinese Proboscidipparion and Plesiohipparion and observations on their Eurasian and African relatives. Vert. PalSin. 53, 73–92.
Bernor, R. L., Tobien, H., Hayek, L.-A. C., and Mittmann, H.-W. (1997). Hippotherium primigenium (Equidae, Mammalia) from the late Miocene of Höwenegg (Hegau, Germany). Andrias 10, 1–230.
Bernor, R. L., Wang, S., Liu, Y., Chen, Y., and Sun, B. (2018). Shanxihippus dermatorhinus (New Gen.) with comparisons to Old World hipparions with specialized nasal apparati. Riv. Ital. Paleontol. Stratigraf. 124, 361–386.
Bertram, J. E. A., and Gutmann, A. (2009). Motions of the running horse and cheetah revisited: fundamental mechanics of the transverse and rotary gallop. J. R. Soc. Interface 6, 549–559. doi: 10.1098/rsif.2008.0328
Biewener, A. A. (1998). Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. B 120, 73–87. doi: 10.1016/S0305-0491(98)00024-8
Biknevicius, A. R., Mullineaux, D. R., and Clayton, H. M. (2006). Locomotor mechanics of the tölt in Icelandic horses. Am. J. Vet. Res. 67, 1505–1510. doi: 10.2460/ajvr.67.9.1505
Biknevicius, A. R., and Reilly, S. M. (2006). Correlation of symmetrical gaits and whole body mechanics: debunking myths in locomotor biomechanics. J. Exp. Zool. 305A, 923–934. doi: 10.1002/jez.a.332
Camp, C. L., and Smith, N. (1942). Phylogeny and functions of the digiial ligaments of the horse. Mem. Univ. Calif. Berkeley 13,1–73.
Cantalapiedra, J. L., Prado, J. L., Fernandez, M. H., and Alberdi, M. T. (2017). Decoupled ecomorphological evolution and diversification in Neogene-Quaternary horses. Science 355, 627–630. doi: 10.1126/science.aag1772
Clayton, H. M. (2004). The Dynamic Horse: a Biomechanical Guide to Equine Movement and Performance. Mason, MI: Sport Horse Publications.
Cooper, K. L., Sears, K. E., Uygur, A., Maier, J., Baczkowski, K. S., Brosnahan, M., et al. (2014). Patterning and post-patterning modes of evolutionary digit loss in mammals. Nature 511, 41–45. doi: 10.1038/nature13496
Damuth, J., and Janis, C. M. (2011). On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. 86, 733–758. doi: 10.1111/j.1469-185X.2011.00176.x
Deng, T., Li, Q., Tseng, J., Takeuchi, G. T., Wang, Y., Xie, G., et al. (2012). Locomotive implication of a Pliocene three-toed horse skeleton from Tibet and its paleo-altimetry significance. Proc. Natl. Acad. Sci. U.S.A. 109,7374–7378. doi: 10.1073/pnas.1201052109
Der Sarkission, C. D., Vilstrup, J. T., Schubert, M., and Seguin-Orlando, E.me, D. 10 others (2015). Mitochondrial genomes reveal the extinct Hippidion as an outgroup to all living equids. Biol. Lett. 11:20141058. doi: 10.1098/rsbl.2014.1058
Eronen, J. T., Ataabadi, M. M., Micheels, A., Karme, A., Bernor, R. L., and Fortelius, M. (2009). Distribution history and climatic controls of the Late Miocene Pikermian chronofauna. Proc. Natl. Acad. Sci. U.S.A. 106, 11867–11871. doi: 10.1073/pnas.0902598106
Eronen, J. T., Fortelius, M., Portmann, F. T., Puolamäki, K., and Janis, C. M. (2012). Neogene aridification of the Northern Hemisphere. Geology 40, 823–826. doi: 10.1130/G33147.1
Famoso, N. A., Davis, E. B., Feranec, R. S., Hopkins, S. S. B., and Price, S. A. (2016). Are hypsodonty and occlusal enamel complexity evolutionarily correlated in ungulates? J. Mamm. Evol. 23, 43–47. doi: 10.1007/s10914-015-9296-7
Hulbert, R. C., and MacFadden, B. J. (1991). Morphological transformation and cladogenesis at the base of the adaptive radiation of Miocene hypsodont horses. Am. Mus. Nov. 3000, 1–61.
Hussain, S. T. (1975). Evolutionary and functional anatomy of the pelvic limb in fossil and Recent Equidae (Perissodactyla, Mammalia). Anat. Histol. Embryol. 4, 179–222. doi: 10.1111/j.1439-0264.1975.tb00636.x
Ingolfsdottir, I. B. (2013). Training im Gelände – Sich die Natur zu Nutze machen. Eidfaxi 4, 70–75.
Janis, C. M. (2007). “The horse series,” in Icons of Evolution, ed B. Regal (Westport, CT: Greenwood Press), 257–280.
Janis, C. M., Damuth, J., and Theodor, J. M. (2004a). The species richness of Miocene browsers, and implications for habitat type and primary productivity in the North American grassland biome. Palaeogeogr. Palaeoclimatol. Palaeoecol. 207, 371–398. doi: 10.1016/j.palaeo.2003.09.032
Janis, C. M., Errico, P., and Mendoza, M. (2004b). Morphological indicators of cursoriality in equids: legs fail to support the “arms race.” J. Vertebr. Paleontol. 24 (Suppl. 3), 75A.
Janis, C. M., Hulbert, R., and Mihlbachler, M. (2008). “Addendum to Volume 1,” in Evolution of Tertiary Mammals of North America, Vol. 2, Small Mammals, Edentates, and Marine Mammals, eds C. M. Janis, G. F. Gunnell, and M. Uhen (Cambridge: Cambridge University Press), 645–693.
Janis, C. M., and Wilhelm, P. W. (1993). Were there mammalian pursuit predators in the Tertiary? Dances with wolf avatars. J. Mamm. Evol. 1, 103–125. doi: 10.1007/BF01041590
Jones, K. E. (2016). New insights on equid locomotor evolution from the lumbar region of fossil horses. Proc. R. Soc. Lond. B Bio. 283:20152947. doi: 10.1098/rspb.2015.2947
Kelly, T. S. (1995). New Miocene horses from the Caliente Formation, Cuyama Valley Badlands, California. Nat. Hist. Mus. L. A. Count. Contrib. Sci. 455, 1–33.
Kelly, T. S. (1998). New Middle Miocene equid crania from California and their implications for the phylogeny of the Equinae. Nat. Hist. Mus. L. A. Count. Contrib. Sci. 473, 1–44.
Lindsay, E. H., Opdyke, N. D., and Johnson, N. M. (1980). Pliocene dispersal of the horse Equus and late Cenozoic mammal dispersal events. Nature 287, 135–138. doi: 10.1038/287135a0
Lister, A. M. (2014). Behavioural leads in evolution: evidence from the fossil record. Biol. J. Linn. Soc. 112, 315–331. doi: 10.1111/bij.12173
MacFadden, B. J. (1992). Fossil Horses: Systematics, Paleobiology, and Evolution of the Family Equidae. Cambridge, UK: Cambridge University Press.
MacFadden, B. J. (1998). “Equidae,” in Evolution of Tertiary Mammals in North America, Vol 1, Terrestrial Carnivores, Ungulates, and Ungulate-like Mammals, eds C. M. Janis, K. M. Scott, and L. L. Jacobs (Cambridge: Cambridge University Press), 537–559.
MacFadden, B. J. (2013). Dispersal of Pleistocene Equus (family Equidae) into South America and calibration of GABI 3 based on evidence from Tarija, Bolivia. PLoS ONE 8:e59277. doi: 10.1371/journal.pone.0059277
Maguire, K. C., and Stigall, A. L. (2008). Paleobiogeography of Miocene Equinae of North America: a phylogenetic biogeographic analysis of the relative roles of climate, vicariance, and dispersal. Palaeogeogr. Palaeoclimatol. Palaeoecol. 267, 175–184. doi: 10.1016/j.palaeo.2008.06.014
Maguire, K. C., and Stigall, A. L. (2009). Using ecological niche modeling for quantitative biogeographic analysis: a case study of Miocene and Pliocene Equinae in the Great Plains. Paleobiology 35, 587–611. doi: 10.1666/0094-8373-35.4.587
Matthew, W. D. (1926). The evolution of the horse; a record and its interpretation. Quart. Rev. Biol. 1, 139–185. doi: 10.1086/394242
McGuigan, M. P., and Wilson, A. M. (2003). The effect of gait and digital flexor muscle activation on limb compliance in the forelimb of the horse Equus caballus. J. Exp. Biol. 206, 1325–1336. doi: 10.1242/jeb.00254
McHorse, B. K., Biewener, A. A., and Pierce, S. E. (2017). Mechanics of evolutionary digit reduction in fossil horses (Equidae). Proc. R. Soc. Lond. B. Bio. 283:20171174. doi: 10.1098/rspb.2017.1174
Mihlbachler, M. C., Rivals, F., Solounias, N., and Semprebon, G. M. (2011). Dietary change and evolution of horses in North America. Science 331, 1178–1181. doi: 10.1126/science.1196166
Minetti, A. E., Ardig,ò, L. P., Reinach, E., and Saibene, F. (1999). The relationship between mechanical work and energy expenditure of locomotion in horses. J. Exp. Biol. 202. 2329–2338.
Nickel, R., Schummer, A., Seiferle, E., Wilkens, H., Wille, K.-H., and Frewein, J. (1986). The Anatomy of the Domestic Animals, Vol 1. New York, NY: Springer-Verlag.
Nicodemus, M. C., and Clayton, H. M. (2003). Temporal variables of four-beat, stepping gaits of gaited horses. Appl. Anim. Behav. Sci. 80, 122–142. doi: 10.1016/S0168-1591(02)00219-8
Osborn, H. F. (1918). Equidae of the oligocene, miocene, and pliocene of North America. Iconographic type revision. Mem. Am. Mus. Nat. Hist. N. Ser. 2, 1–326.
O'Sullivan, J. A. (2008). “Evolution of the proximal third phalanx in Oligocene-Miocene equids, and the utility of phalangeal indices in phylogeny reconstruction,” in Mammalian Evolutionary Morphology: A Tribute to Fred Szalay, eds E. J. Sargis, and M. Dagosto (New York, NY: Springer), 159–165.
Pagnac, D. (2006). Scaphohippus, a new genus of horse (Mammalia: Equidae) from the barstow formation of California. J. Mamm. Evol. 13, 37–61. doi: 10.1007/s10914-005-9002-2
Parker, A. K., McHorse, B. K., and Pierce, S. E. (2018). Niche modeling reveals a lack of broad-scale habitat partitioning in extinct horses of North America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 511, 103–118. doi: 10.1016/j.palaeo.2018.07.017
Pennycuick, C. J. (1975). On the running of the gnu (Connochaetes taurinus) and other animals. J. Exp. Biol. 63, 775–779.
Promerová, M., Andersson, L. S., Juras, R., Penedo, M. C. T., and Reissmann, M. 16 others (2014). Worldwide frequency distribution of the ‘Gait Keeper’ mutation in the DMRT3 gene. Anim. Genet. 45, 274–282. doi: 10.1111/age.12120
Qiu, Z. X., Huang, W. L., and Guo, Z. H. (1987). The Chinese hipparionine fossils. Palaeontol. Sin. New Ser. C 25, 1–243.
Reilly, S. M., and Biknevicius, A. R. (2007). Posture, gait and the ecological relevance of locomotor costs and energy-saving mechanisms in tetrapods. Zoology 110, 271–289. doi: 10.1016/j.zool.2007.01.003
Renders, E. (1984). The gait of Hipparion sp. from fossil footprints in Laetoli, Tanzania. Nature 308, 179–181. doi: 10.1038/308179a0
Salesa, M. J., Sánchez, I. M., and Morales, J. (2004). Presence of the Asian horse Sinohippus in the Miocene of Europe. Acta Palaeontol. Pol. 49, 189–196.
Semprebon, G. M., Rivals, F., Solounias, N., and Hulbert, R. C. Jr. (2016). Paleodietary reconstruction of fossil horses from the Eocene through Pleistocene of North America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 442, 110–127. doi: 10.1016/j.palaeo.2015.11.004
Shoemaker, L., and Clauset, A. (2014). Body mass evolution and diversification within horses (family Equidae). Ecol. Lett. 17, 211–220. doi: 10.1111/ele.12221
Shotwell, J. A. (1961). Late Tertiary biogeography of horses in the northern Great Basin. J. Paleontol. 35, 203–217.
Sondaar, P. Y. (1968). The osteology of the manus of fossil and Recent Equidae with special reference to phylogeny and function. Verh. K. Ned. Akad. Wet. 25, 1–76.
Stashak, T. S. (2006). Practical Guide to Lameness in Horses. Ames, IA: Blackwell Publishing Professional.
Stirton, R. A. (1940). Phylogeny of North American Equidae. Univ. California Pub. Bull. Dept. Geol. Sci. 25, 165–198.
Sun, B., Zhang, X., Liu, Y., and Bernor, R. L. (2018). Sivalhippus ptychodus and Sivalhippus platyodus (Perissodactyla, Mammalia) from the Late Miocene of China. Riv. Ital. Paleontol. Stratigraf. 124, 1–22.
Taylor, C. R., Heglund, N. C., and Maloiy, G. M. O. (1982). Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 1–12.
Thomason, J. J. (1985). Estimation of locomotory forces and stresses in the limb bones of Recent and extinct equids. Paleobiology 11, 209–220. doi: 10.1017/S0094837300011519
Thomason, J. J. (1986). The functional morphology of the manus in tridactyl equids Merychippus and Mesohippus: paleontological inferences from neontological models. J. Vertebr. Paleontol. 6, 143–161. doi: 10.1080/02724634.1986.10011607
Tucker, S. T., Otto, R. E., Joeckel, R. M., and Voorhies, M. R. (2014). The geology and paleontology of Ashfall Fossil Beds, a late Miocene (Clarendonian) mass-death assemblage, Antelope County and adjacent Knox County, Nebraska, USA. Field Guides 36, 1–32. doi: 10.1130/2014.0036(01)
Voorhies, M. R. (1981). Ancient skyfall creates Pompeii of prehistoric animals. Natl. Geogr. 159, 66–75.
Voorhies, M. R. (1994). The cellars of time – paleontology and archaeology in Nebraska. Nebrask. Magaz. Nebrask. Game Park Div. 72, 1–162.
Waldern, N. M., Wiestner, T., Ramseier, L. C., and Weishaupt, M. A. (2015). Comparison of limb loading of Icelandic horses while tölting and trotting at equal speeds. Am. J. Vet. Res. 76, 1031–1040. doi: 10.2460/ajvr.76.12.1031
Wang, S., and Deng, T. (2011). Some evolutionary trends of Equus eisenmannae (Mammalia, Perissodactyla) in the stratigraphic sequence of Longdan, China, in comparison to modern Equus. J. Vertebr. Paleontol. 31, 1356–1365 doi: 10.1080/02724634.2011.611203
Wilson, A. M., McGuigan, M. P., Su, A., and van den Bogert, A. J. (2001). Horses damp the spring in their step. Nature 414, 895–899. doi: 10.1038/414895a
Wolf, D., Bernor, R. L., and Hussain, S. T. (2013). A systematic, biostratigraphic, and paleobiogeographic reevaluation of the Siwalik hipparionine horse assemblage from the Potwar Plateau, Northern Pakistan. Palaeontogr. Abt. A. 300, 1–115. doi: 10.1127/pala/300/2013/1
Woodburne, M. O. (2007). Phyletic diversification of the Cormohipparion occidendale complex (Mammalia: Perissodactyla, Equidae), late Miocene, North America, and the origin of the Old World Hippotherium datum. Bull. Am. Mus. Nat. Hist. 306, 1–138. doi: 10.1206/0003-0090(2007)306[1:PDOTCO]2.0.CO;2
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. (2001). Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. doi: 10.1126/science.1059412
Keywords: Equidae, Hipparionini, Equini, paleobiology, locomotion, evolution, monodactyly
Citation: Janis CM and Bernor RL (2019) The Evolution of Equid Monodactyly: A Review Including a New Hypothesis. Front. Ecol. Evol. 7:119. doi: 10.3389/fevo.2019.00119
Received: 09 January 2019; Accepted: 22 March 2019;
Published: 12 April 2019.
Edited by:
Leonardo Dos Santos Avilla, Universidade Federal do Estado do Rio de Janeiro, BrazilReviewed by:
Spencer G. Lucas, New Mexico Museum of Natural History and Science, United StatesDavid Marjanović, Museum für Naturkunde, Germany
Copyright © 2019 Janis and Bernor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine M. Janis, christine_janis@brown.edu
 Christine M. Janis
Christine M. Janis Raymond L. Bernor
Raymond L. Bernor