- 1Behavioural and Clinical Neuroscience Institute and Department of Psychiatry, University of Cambridge School of Clinical Medicine, Cambridge, United Kingdom
- 2Department of Biomedical and Neuromotor Sciences (DIBINEM), University of Bologna, Bologna, Italy
- 3Clinical Neuropsychology, Cognitive Disorders and Dyslexia Unit, Department of Neuro-Motor Diseases, Azienda Unità Sanitaria Locale - IRCCS, Reggio Emilia, Italy
- 4Department of Medicine and Surgery, University of Parma, Parma, Italy
- 5IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy
Sleep paralysis (SP) is a condition where a person is paralyzed upon waking or falling asleep. SP afflicts ~20% of people, and is also one of the typical symptoms in narcolepsy. During SP the sleeper may experience hallucinations. Unsurprisingly, SP is associated with great fear globally. To date, there are no published clinical trials or outcome data for treating this condition. However, few non-pharmacological interventions have been proposed, including cognitive behavioral approaches, and case studies showing clinical amelioration with auto-hypnosis and Meditation-Relaxation (MR) therapy. The latter for instance showed positive preliminary results; when applied for 8 weeks it reduced SP frequency and anxiety/worry symptoms. With this paper we aimed to evaluate, with a small-scale pilot study, the efficacy of MR therapy for SP in patients with narcolepsy. Ten patients with narcolepsy and SP were enrolled in the study. Notably, MR therapy (n = 6), applied for 8 weeks, resulted in a dramatic decrease in the number of days SP occurred (50% reduction); and the total number of SP episodes (54% reduction) in the last month of the study (demonstrated by large within-group effect sizes); unlike the control intervention (deep breathing) (n = 4). These findings are preliminary and exploratory given the small sample. Nonetheless, they represent the first proof of concept at providing empirically-guided insights into the possible efficacy of a novel treatment for frequently occurring SP. Although the study was conducted in patients with narcolepsy we cautiously suggest that the findings may generalize to individuals with isolated SP.
Introduction
Sleep paralysis (SP) is a state of atonia—skeletal muscles paralysis—occurring at sleep onset and offset. While temporarily immobilized, the person is acutely aware of his/her surroundings (i.e., semi or fully conscious) (1–3). SP commonly occurs in narcolepsy; a sleep disorder involving excessive daytime sleepiness, cataplexy and other untimely manifestations of rapid-eye-movement (REM) sleep such hypnagogic hallucinations and SP (4). SP is also prevalent in the general population, afflicting ~20% of people (1, 5, 6).
Hypnogogic or hypnopompic hallucinations may occur during SP and can involve all sensory modalities (7). Strikingly, SP experiencers often report being terrorized by dangerous bedroom intruders (8, 9). Supernatural interpretations of SP are found worldwide and often reflect the cultural background of the population in question.
In terms of neurophysiology, SP represents a REM sleep parasomnia reflecting the dissociation between REM sleep and wakefulness, with the concurrent presence of REM sleep muscle atonia, wakefulness consciousness, and, eventually REM dream mentation (1). SP occurs at the transition between wakefulness and REM sleep in narcolepsy patients—or in otherwise healthy subjects (10). Polysomnographic studies disclosed the co-occurrence of wakefulness features and REM sleep during SP episodes (11, 12). SP ranges in duration from a few seconds up to 20 min, although under paralysis patients are not accurate in such subjective judging of the passing of time (13).
SP in the general population may be triggered by sleep deprivation (14), and is more frequent in psychiatric conditions like post-traumatic stress disorder (15). Unsurprisingly, SP is linked to excessive fear, which persists after continual exposure to the event (16, 17).
Notwithstanding the trepidation and anxiety associated with the experience, to date there are no published clinical trials or outcome data for treating this condition. However, few non-pharmacological interventions have been proposed including cognitive behavioral approaches (18, 19). Moreover, case studies have shown clinical amelioration with auto-hypnosis (13), and Meditation-Relaxation (MR) therapy (20). Only a limited number of patients report a severe enough condition that would allow for the off-label use of antidepressants (21, 22). Thus, most sufferers would benefit from a non-pharmacological intervention.
MR therapy—a simple (non-invasive) and direct 4-step psychological intervention—was recently proposed for SP (20). The aim of this pilot study was to explore the preliminary efficacy of MR therapy for SP (applied for 8 weeks) in patients with narcolepsy, compared to deep breathing techniques. A secondary aim was to examine features of SP in narcolepsy and its link to psychopathology (mood/anxiety).
Methods
Participants
The study was conducted at the Center for Narcolepsy of the University of Bologna/IRCCS Istituto delle Scienze Neurologiche di Bologna, Italy and approved by the ethics committee at the CE-AVEC Independent Ethical Committee, Area Vasta Emilia Centro. Patients provided written consent. Participants met criteria for narcolepsy (type 1 and 2) based on polysomnography, Multiple Sleep Latency Test (MLST) and CSF hypocretin level testing. Patients had to have experienced SP at least four times in the last month. The study included ten patients (female = 40%; age: M = 27.8, SD = 12.2) (Two patients were <18 years old; one turned 18 during the study [in both cases written parental approval was obtained]).
Measures
SP Assessment
SP was assessed using the Sleep Paralysis Experiences and Phenomenology Questionnaire (SP-EPQ) (23–25).
Daily Journal (Outcome Variables)
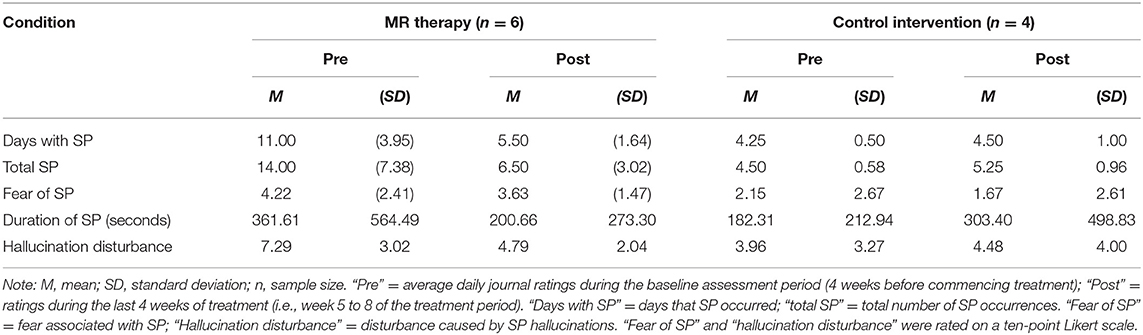
Each day during the study (12 weeks), participants rated their SP occurrence, perceived duration (seconds), fear associated with SP, and disturbance caused by hallucinations (on a ten-point scale).
Baseline Measures of Mood/Anxiety
Participants completed the following validated measures: the Beck Depression Inventory-II (BDI-II) (26), Spielberger State-Trait Anxiety Inventory (STAI-S/T) (27), and Penn State Worry Questionnaire (PSWQ) (28). The Italian versions were completed (29–31).
Intervention
MR therapy is a psychological treatment for SP, comprised of the following steps applied directly during the attack: Step I: Reappraisal of the meaning of the attack; Step II: psychological and emotional distancing; Step III: inward focused-attention meditation; Step IV: Muscle relaxation [for details see (20)]. The control intervention was identical, except participants engaged in deep breathing instead; entailing slow deep breaths, while repeatedly counting from 1 to 10. This is an active control (breathing-distraction exercise) rather than a placebo.
Procedure
SP experiencers were recruited at the Outpatient Clinic for Narcolepsy and assigned to either the MR therapy or control group (open label study design). Participants were orally administered the SP-EPQ to assess their SP (e.g., frequency and features), and were instructed to keep daily a journal for 4 weeks as a baseline assessment of SP occurrence, duration and emotions (see above) and email this to the researcher each week. After the 4 weeks, participants completed mood/anxiety questionnaires and were taught the therapy techniques (i.e., practiced them with the experimenter), and instructed to rehearse these during ordinary wakefulness (simulating a SP attack), twice a week for 15 min. These instructions (e.g., treatment techniques) were administered in person or via video call. Participants then initiated the treatment which lasted 8 weeks. They kept the daily journal during the entire study period (12 weeks).
Statistics
Exploration of the link between SP frequency and hallucinatory-induced disturbance and mood/anxiety symptoms were analyzed using a Pearson correlation test. Independent sample t-tests were used to analyze baseline group differences on outcome variables. Specific hypotheses pertaining to MR therapy and control intervention were analyzed using focused directional paired sample t-tests.
For test of a priori hypotheses (i.e., one-tailed t-tests), the Benjamini and Hochberg's (32) false discovery rate (FDR) was applied to correct for potential Type I errors. The FDR was set at q <0.15, based on guidelines in the field (33). The corrected significance level/critical value was 0.045. Displayed P-values are raw; designated significant/non-significant after applying the correction (34). Exploratory/baseline analyses were not corrected for multiple comparisons, which is unnecessary (35).
The distribution of residuals was checked using the Shapiro-Wilk test and Q-Q plots for all measures. Residuals sometimes departed from normality. Given the F/t-test is overall robust to violations (36), the data were analyzed with those caveats.
Results
Baseline/Pre-treatment Period (Initial 4 Weeks)
Features of SP
The patients (n = 10) experienced SP on average 8.30 days (SD = 4.57) total, and 10.20 (SD = 7.38) SP episodes in the last month (i.e., during the baseline period). They reported being paralyzed on average 289.89 sec (SD = 448.01). Patients tended to hallucinate during SP (on average in 66% [SP with hallucinations/total SP] of cases in the last month). SP hallucinations often occurred upon awakening from sleep (51%), and less frequently upon falling asleep (14%); a proportion of SP episodes occurred during “sleep” (i.e., neither at onset nor offset; 24%).
SP, Hallucinations and Mood
The number of SP episodes in the last month did not correlate with depression, anxiety and worry symptoms (i.e., during the baseline period). Disturbance caused by SP hallucinations correlated with depression (r = 0.69, p = 0.039) and worry symptoms (r = 0.69, p = 0.039) unlike anxiety (there was a trend for trait anxiety: r = 0.58, p = 0.099) (one patient did not complete these questionnaires: n = 9).
Treatment
Baseline Difference
Participants did not differ on outcome variables (fear, perceived duration of SP, and disturbance caused by hallucinations) except that participants in the MR therapy group reported a greater number of days with SP (t5.24 = 4.14, p = 0.008) and total number of SP (t5.09 = 3.14, p = 0.025) in the last month (during the baseline period).
MR Therapy Pre-post
There was a reduction in the number of days with SP (t5 = 4.68, p = 0.0025, one-tailed, d = 1.91) and total number of SP (t5 = 3.86, p = 0.006, one-tailed, d = 1.57) in the last month pre-post MR therapy. The average fear of SP (t5 = 0.57, p = 0.298, one-tailed, d = 0.23) and perceived duration of SP (t5 = 1.33, p = 0.121, one-tailed, d = 0.54) in the last month were unchanged; there was a notable tendency toward reductions in the disturbance caused by hallucinations (t5 = 2.01, p = 0.051, one-tailed, d = 0.82).
In the control condition, the number of days with SP (t3 = −0.52, p = 1.28, one-tailed, d = −0.26) and total number of SP (t3 = −1.57, p = 0.44, one-tailed, d = −0.78) in the last month; fear (t3 = 2.80, p = 0.136, one-tailed, d = 1.40) and perceived duration (t3 = −0.83, p = 0.936, one-tailed, d = −0.41) of SP, and disturbance caused by hallucinations in the last month (t3 = −0.92, one-tailed, p = 0.850, d = −0.46) were unchanged [Means and standard deviations are displayed in Table 1; key findings are shown in Figure 1; data points plotted for individual patients [pre-post treatment] are displayed in Figure 2].
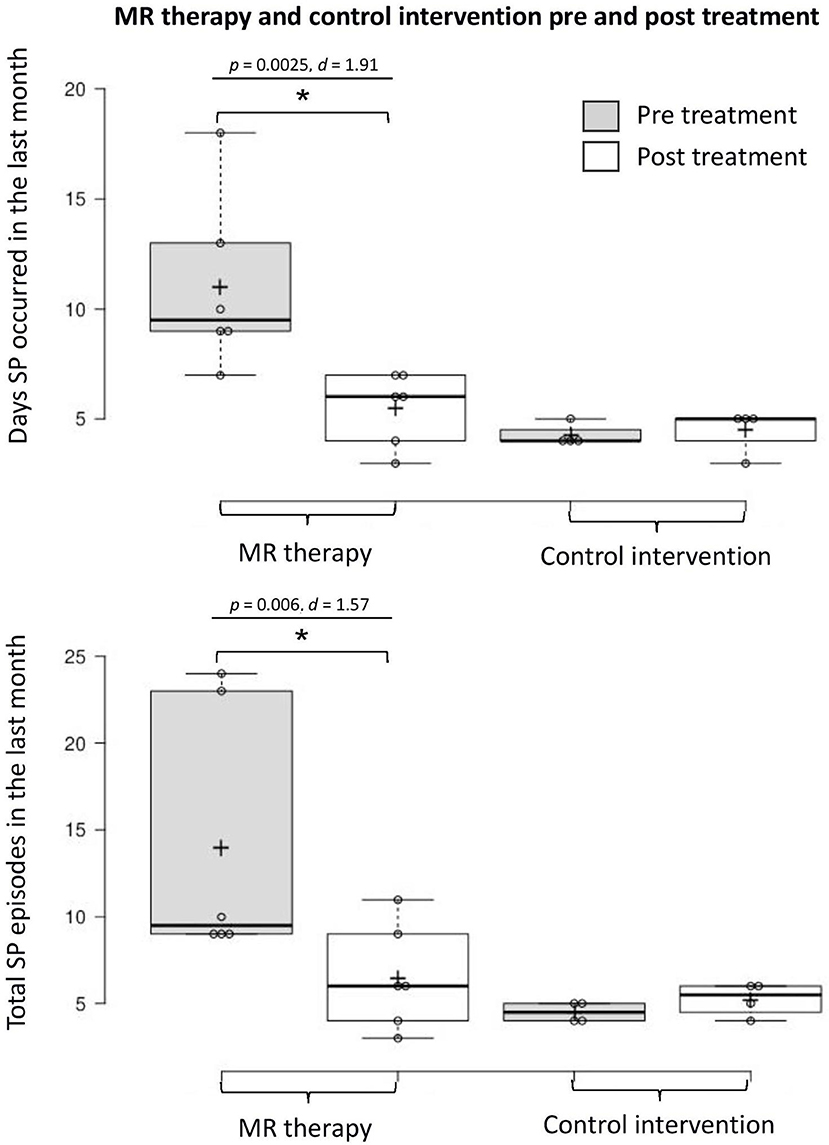
Figure 1. Days with SP and total number of SP in the last month pre-post MR therapy and control intervention (deep breathing). “Pre treatment” reflects the baseline assessment period (4 weeks before commencing treatment); “Post treatment” reflects the last 4 weeks of treatment (week 5 to 8 of the treatment period). *Denotes a statistically significant result.
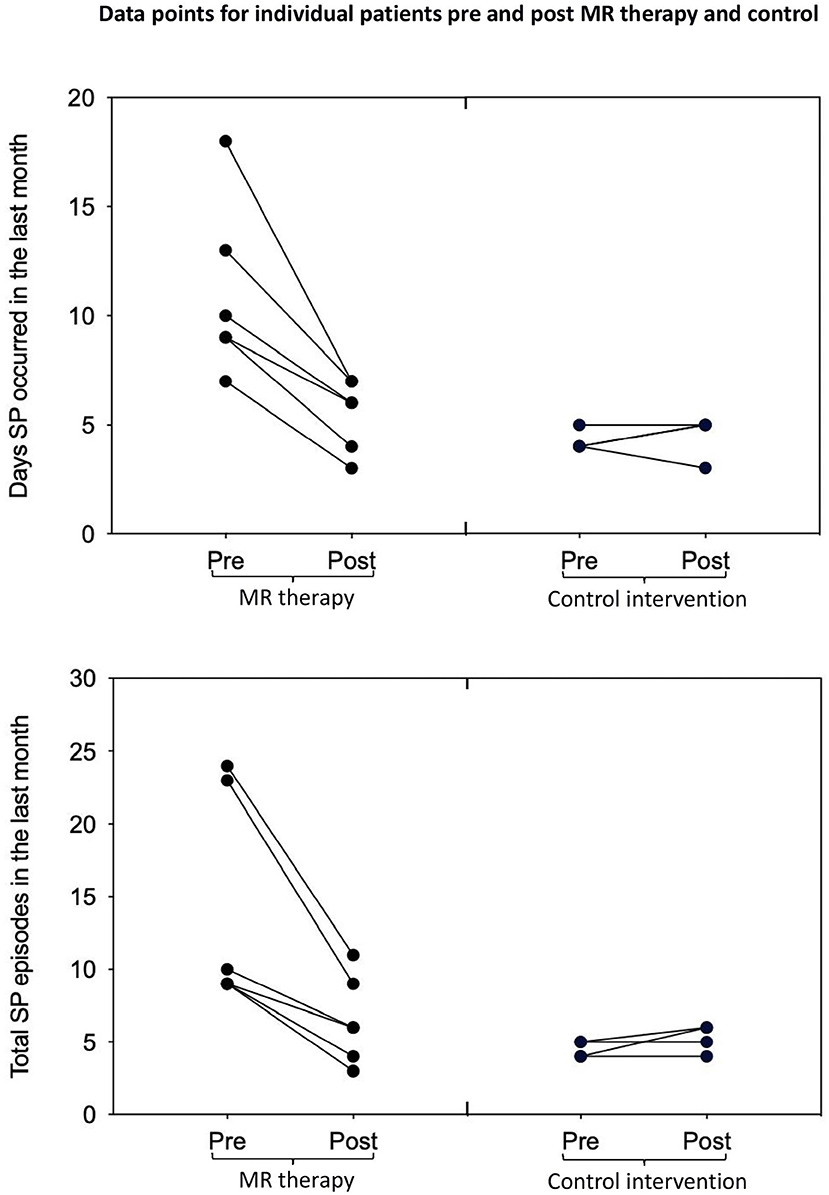
Figure 2. Days with SP and total number of SP in the last month pre-post MR therapy and control intervention (deep breathing). “Pre treatment” reflects the baseline assessment period (4 weeks before commencing treatment); “Post treatment” reflects the last 4 weeks of treatment (week 5 to 8 of the treatment period).
Discussion
This small-scale pilot study yields novel findings with clinical implications. MR therapy resulted in a dramatic decrease in the number of days SP was experienced (50% reduction), and total number of episodes (54% reduction) in the last month of the study (illustrated by large within-group effect sizes). There was also a noteworthy (p = 0.05) trend toward reductions in the disturbance caused by SP hallucinations (a 34% reduction in self-report ratings, with a large effect size). Given the small sample size, p-values should be interpreted cautiously, and effect sizes should take priority. These effects were not observed in the control intervention where the occurrence of SP and associated negative emotional states were unchanged. Overall, these preliminary findings suggest that MR therapy potentially constitutes a promising treatment option for SP; pending confirmation in randomized clinical trials.
That MR therapy was applied by the patients in their home environment is notable. Given the simplicity of MR therapy, it lends itself to internet- and smartphone-based delivery; e.g., where therapy instructions are administered online, and remotely overseen by a clinician, who can intervene promptly when necessary. As noted, in this study, in some cases for logistic reasons therapy was delivered via video call. In brief, this low-cost and accessible intervention may facilitate early intervention (prevent SP from becoming chronic and possibly spiral into psychopathology), and is potentially suitable for poorly resourced settings and low-income countries with restricted access to health care. This approach may be of valuable utility also in periods such as the current COVID-19 pandemic when face-to-face interventions are limited.
In the current study, consistent with research in general populations, disturbance caused by SP was associated with elevated psychopathology (depressive and worry) symptomatology. These findings are of clinical value. They suggest that SP-associated psychological disturbance may contribute to overall emotional instability in narcolepsy (37). It is also conceivable that elevated psychopathology symptoms might increase the tendency to catastrophize SP hallucinations. As such, applying psychological treatments to specifically target catastrophic cognitions associated with SP-related fears (e.g., combined with direct SP treatments) may help improve these patients' emotional well-being (reduce distress) and effectively their global functioning (38).
This novel venture sheds light on the experience of SP in narcolepsy. Reminiscent of the general population, patients' self-estimated duration of SP was ~5 min, occurred most often at sleep offset, and a comparable proportion of individuals hallucinated during the event [e.g., 66 vs. 72% in the general Italian population, see (25)]. Taken together, SP in narcolepsy and the general population appear to be phenomenologically indistinguishable, and further studies are needed to directly compare their features.
The patients in this study relied on a daily journal to rate their SP occurrence, duration and emotions which constitutes a strength; i.e., that they kept a journal not only during the treatment (8 weeks), but also prior during baseline assessment (4 and 12 weeks total). In contrast, retrospective accounts of such physiological and psychological states may lack precision. The study has limitations. Since it was conducted in narcolepsy patients the findings may possibly generalize to isolated SP patients; this should be explored further (given that SP in narcolepsy, unlike ISP, is within the context of hypocretin deficiency typical of narcolepsy). Also, while the reduction in SP following MR therapy was striking (as noted, a 54% monthly reduction), we cannot rule out that pre-existing higher frequency/severity of SP may have accounted for its superiority relative to the control intervention. Indeed, future research (randomized controlled trials) should actively match groups based on clinical severity to rule out a threshold effect; that we did not do so constitutes a limitation. In this study, patients were assigned to the two groups in a non-randomized, open label fashion; however, future studies should ensure proper allocation concealment to prevent potential selection bias. Finally, future sufficiently powered studies should assess the effect of therapy on a greater number of outcome variables (e.g., related to hallucinations and mood). We hasten to add that these findings are preliminary given the small sample. Nonetheless, they represent the first attempt at providing (empirically-guided) insights into the possible efficacy of a novel treatment for chronic SP—a condition all too often overlooked by clinicians and researchers alike.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the CE-AVEC Independent Ethical Committee, Area Vasta Emilia Centro. Written informed consent to participate in this study was provided by the participants and/or the participants' legal guardian/next of kin.
Author Contributions
BJ designed the study, performed the data analysis, and wrote the manuscript. AZ designed the study, analyzed the data, and edited the manuscript. LM recruited and tested patients (e.g., administered the therapy), prepared the data for analysis, and edited the manuscript. FP recruited patients and edited the manuscript. GP designed the study, recruited patients, and edited the manuscript. MF recruited patients, and reviewed the manuscript. CF recruited patients and reviewed the manuscript. All authors read and approved the submitted version.
Conflict of Interest
BJ will receive royalties from Cambridge University Press commencing 2020. GP has served as consultant for UCB, Bioprojet, Jazz, Idorsia.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. American Academy of Sleep Medicine. International Classification of Sleep Disorders. Diagnostic and Coding Manual (3rd ed.). Darien, IL: American Academy of Sleep Medicine (2014).
3. Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science (4th ed). New York, NY: McGraw-Hill (2000).
4. Bassetti CL, Adamantidis A, Burdakov D, Han F, Gay S, Kallweit U, et al. Narcolepsy—clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. (2019) 15: 519–39. doi: 10.1038/s41582-019-0226-9
5. Jalal B, Hinton DE. Lifetime presence and rates of sleep paralysis in Denmark of ethnic Danes and non-ethnic Danes. Psychia Clin Neurosci. (2016) 70: 253. doi: 10.1111/pcn.12385
6. Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev. (2011) 15:311–5. doi: 10.1016/j.smrv.2011.01.007
7. Jardri R, Bartels-Velthuis AA, Debbané M, Jenner JA, Kelleher I, Dauvilliers Y, et al. From phenomenology to neurophysiological understanding of hallucinations in children and adolescents. Schizophr Bull. (2014) 40:S221–32. doi: 10.1093/schbul/sbu029
8. Cheyne JA, Girard TA. The body unbound: vestibular–motor hallucinations and out-of-body experiences. Cortex. (2009) 45:201–15. doi: 10.1016/j.cortex.2007.05.002
9. Jalal B, Ramachandran VS. Sleep paralysis and “the bedroom intruder”: the role of the right superior parietal, phantom pain and body image projection. Med Hypotheses. (2014) 83:755–7. doi: 10.1016/j.mehy.2014.10.002
10. Takeuchi T, Miyasita A, Sasaki Y, Inugami M, Fukuda K. Isolated sleep paralysis elicited by sleep interruption. Sleep. (1992) 15:217–25. doi: 10.1093/sleep/15.3.217
11. Pizza F, Moghadam KK, Franceschini C, Bisulli A, Poli F, Ricotta L, et al. Rhythmic movements and sleep paralysis in narcolepsy with cataplexy: a video-polygraphic study. Sleep Med. (2010) 11:423–5. doi: 10.1016/j.sleep.2010.01.005
12. Terzaghi M, Ratti PL, Manni F, Manni R. Sleep paralysis in narcolepsy: more than just a motor dissociative phenomenon? Neurologic Sci. (2012) 33:169–72. doi: 10.1007/s10072-011-0644-y
13. Nardi TJ. Treating sleep paralysis with hypnosis. Int J Clin Experiment Hypnosis. (1981) 29:358–65. doi: 10.1080/00207148108409169
14. McCarty DE, Chesson AL Jr. A case of sleep paralysis with hypnopompic hallucinations. J Clin Sleep Med. (2009) 5:83–4. doi: 10.5664/jcsm.27398
15. Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compreh Psychia. (2000) 41:469–78. doi: 10.1053/comp.2000.16568
16. Cheyne JA, Pennycook G. Sleep paralysis postepisode distress: modeling potential effects of episode characteristics, general psychological distress, beliefs, and cognitive style. Clin Psycholog Sci. (2013) 1:135–48. doi: 10.1177/2167702612466656
17. Jalal B, Hinton DE. Rates and characteristics of sleep paralysis in the general population of Denmark and Egypt. Cult Med Psychia. (2013) 37:534–48. doi: 10.1007/s11013-013-9327-x
18. Hinton DE, Pich V, Chhean D, Pollack MH. “The ghost pushes you down”: sleep paralysis-type panic attacks in a Khmer refugee population. Transcult Psychiatry. (2005) 42:46–78. doi: 10.1177/1363461505050710
19. Sharpless BA, Doghramji K. Sleep Paralysis: Historical, Psychological, and Medical Perspectives. New York, NY: Oxford University Press (2015). doi: 10.1093/med/9780199313808.001.0001
20. Jalal B. How to make the ghosts in my bedroom disappear? Focused-attention meditation combined with muscle relaxation (MR therapy)—a direct treatment intervention for sleep paralysis. Front Psychol. (2016) 7:28. doi: 10.3389/fpsyg.2016.00028
21. Sharpless BA, McCarthy KS, Chambless DL, Milrod BL, Khalsa SR, Barber JP. Isolated sleep paralysis and fearful isolated sleep paralysis in outpatients with panic attacks. J Clin Psychol. (2010) 66:1292–306. doi: 10.1002/jclp.20724
22. Sharpless BA, Grom JL. Isolated sleep paralysis: fear, prevention, and disruption. Behav Sleep Med. (2016) 14:134–9. doi: 10.1080/15402002.2014.963583
23. Jalal B, Eskici HS, Acarturk ZC, Hinton D. Beliefs about sleep paralysis in Turkey: karabasan. Transcultural Psychia. (2020). doi: 10.1177/1363461520909616. [Epub ahead of print].
24. Jalal B, Romanelli A, Hinton DE. Cultural explanations of sleep paralysis in Italy: the pandafeche attack and associated supernatural beliefs. Cult Med Psychia. (2015) 39: 651–64. doi: 10.1007/s11013-015-9442-y
25. Jalal B, Romanelli A, Hinton DE. Sleep paralysis in Italy: frequency, hallucinatory experiences, and other features. Transcultural Psychiatry. (2020) doi: 10.1177/1363461520909609. [Epub ahead of print].
26. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. (1996) doi: 10.1007/978-1-4419-1005-9_441
27. Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI). Palo Alto, CA: Consulting Psychologists Press (1983). doi: 10.1037/t06496-000
28. Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behav Res Thera. (1990) 28:487–95. doi: 10.1016/0005-7967(90)90135-6
29. Morani S, Pricci D, Sanavio E. “Penn State Worry Questionnaire” e “Worry Domains Questionnaire”. Presentazione delle versioni italiane ed analisi di fedeltà. Psicoterapia Cognitiva Comportamentale. (1999) 5: 195–209.
30. Pedrabissi L, Santinello M. Verifica della validità dello STAI forma Y di Spielberger [Verification of the validity of the STAI, Form Y, by Spielberger]. Giunti Organizzazioni Speciali. (1989) 191−192:11–4.
31. Sica C, Ghisi M. The Italian versions of the beck anxiety inventory and the beck depression inventory-II: psychometric properties and discriminant power. In: Lange MA, editor. Leading-Edge Psychological Tests and Testing Research. New York, NY: NOVA Publishers. (2007) p. 27–50.
32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Soc. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
33. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. (2002) 15:870–8. doi: 10.1006/nimg.2001.1037
34. McDonald JH. Handbook of Biological Statistics (3rd ed.). Baltimore, MD: Sparky House Publishing. (2014). p. 254–60. http://www.biostathandbook.com/
35. Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. (2001) 54:343–9. doi: 10.1016/S0895-4356(00)00314-0
36. Blanca M, Alarcón R, Arnau J, Bono R, Bendayan R. Non-normal data: is ANOVA still a valid option? Psicothema. (2017) 29:552–7. doi: 10.7334/psicothema2016.383
37. Fortuyn HA, Mulders PC, Renier WO, Buitelaar JK, Overeem S. Narcolepsy and psychiatry: an evolving association of increasing interest. Sleep Med. (2011) 12:714–9. doi: 10.1016/j.sleep.2011.01.013
Keywords: sleep paralysis, treatment, meditation-relaxation therapy, narcolepsy, proof-of-concept study
Citation: Jalal B, Moruzzi L, Zangrandi A, Filardi M, Franceschini C, Pizza F and Plazzi G (2020) Meditation-Relaxation (MR Therapy) for Sleep Paralysis: A Pilot Study in Patients With Narcolepsy. Front. Neurol. 11:922. doi: 10.3389/fneur.2020.00922
Received: 28 May 2020; Accepted: 17 July 2020;
Published: 12 August 2020.
Edited by:
Paul James Reading, South Tees Hospitals NHS Foundation Trust, United KingdomReviewed by:
Valérie Cochen De Cock, Hôpital général de Beauvais, FranceGuy Leschziner, Guy's and St Thomas' NHS Foundation Trust, United Kingdom
Copyright © 2020 Jalal, Moruzzi, Zangrandi, Filardi, Franceschini, Pizza and Plazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baland Jalal, drbalandjalal@gmail.com
 Baland Jalal
Baland Jalal Ludovico Moruzzi
Ludovico Moruzzi Andrea Zangrandi
Andrea Zangrandi Marco Filardi
Marco Filardi Christian Franceschini
Christian Franceschini Fabio Pizza
Fabio Pizza Giuseppe Plazzi
Giuseppe Plazzi