Assessing Variability in Vascular Response to Cocoa With Personal Devices: A Series of Double-Blind Randomized Crossover n-of-1 Trials
- 1Department of Clinical and Experimental Medicine, Faculty of Health and Medical Sciences, University of Surrey, Guildford, United Kingdom
- 2Department of Biochemical Sciences, Faculty of Health and Medical Sciences, University of Surrey, Guildford, United Kingdom
- 3Department of Nutritional Sciences, School of Life Course and Population Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, United Kingdom
- 4Vascular Department, Surrey and Sussex NHS Healthcare Trust, Redhill, United Kingdom
Controlled clinical intervention studies have demonstrated that cocoa flavanols (CF) can decrease blood pressure and arterial stiffness in healthy humans, although a large variability in the effect size across trials has been reported. In this study, we evaluated the intra- and inter-individual variability of responses to CF in everyday life using a series of n-of-1 trials in healthy free-living individuals with normal blood pressure carrying personal devices. In total, eleven healthy young humans participated in a repeated crossover randomized controlled double-blind n-of-1 trial. On 8 consecutive days, each volunteer consumed on alternating days 6 CF capsules (862 mg CF) on 4 days and 6 matched placebo capsules (P, 0 mg CF/day) on another 4 days in one of the two randomized sequences (CF-P-CF-P-CF-P-CF-P or P-CF-P-CF-P-CF-P-CF). On each day, the capsules were taken at the same time in the morning with breakfast after baseline measurements. Each subject was provided with an upper arm blood pressure monitor and a finger clip that measures pulse wave velocity (PWV). Measurements of blood pressure, heart rate, and PWV were taken at least hourly over 12 h during the day by the participants. On the first 2 days, measurements were performed under supervision to provide training. The overall mixed model analysis showed that CF significantly decreased 12-h systolic blood pressure and PWV by −1.4 ± 0.3 mmHg and −0.11 ± 0.03 m/s, respectively. Peak effects were observed within the first 3 h (1.5 h SBP: −4.9 ± 2.2 mmHg, PWV: −0.32 ± 0.17 m/s) and again after 8 h post-ingestion. Large inter-individual variation in responses was found [intra-cluster correlation coefficients (ICC): 0.41, 0.41]. When analyzing single individuals’ datasets, there was also considerable between-day variation in individual responses that varied greatly between subjects (ICC: 0–0.30, 0–0.22, 0–0.45). Effect sizes inversely correlated with baseline blood pressure values both between- and within-subjects. The data confirm that cocoa can decrease blood pressure and arterial stiffness in everyday life when elevated within the normal range. The large inter- and intra-individual variation in responses calls for more personalized nutritional intervention strategies.
Introduction
Epidemiological data suggest that there is an inverse relationship between the consumption of flavonoid-rich diets and the risk for cardiovascular disease (1–6). Significant associations were also observed for one subclass of flavonoids, the flavanols, and their oligomers, the procyanidins (2, 7). Dietary intervention studies with flavanol and procyanidin-containing foods support this notion, as the consumption of these foods improves arterial endothelial function while lowering blood pressure and arterial stiffness in healthy subjects (8–10). However, the clinical intervention studies were performed in tightly controlled experimental settings, and in particular, effects on blood pressure show large heterogeneity between trials and suggest that baseline blood pressure may play a role in the effect of cocoa on blood pressure (10), As participants’ responses were only measured once at specified times after single or repeated ingestion of interventions in the laboratory, individual responses were not investigated at different days and not during real life. A profound understanding of inter- and intra-individual variability of responses in real life is an important area of research in a world that aims at personalized nutrition and medicine.
In the so-called n-of-1 study design, individuals are exposed to interventions and control multiple times (11, 12). It has been proposed recently as a powerful tool allowing to study small effects even in fewer subjects as inter-individual variability is reduced by repeatedly performing interventions in the same individuals. More importantly, it allows to study the variability of responses in individual people to identify “responders” and “non-responders” and also to understand determinants of the response (13). Some authors have argued that n-of-1 trials may be the ultimate strategy for individualizing medicine (13). The overall aim of this study was to evaluate the intra- and inter-individual variability in vascular responses to cocoa flavanols using personal devices and n-of-1 study design.
Materials and Methods
Study Design and Participants
The study participants (n = 11) were healthy male and female adult subjects (<45 years) with normal blood pressure and without history, signs, or symptoms indicative of cardiovascular disease, including previous myocardial infarction, stroke, and peripheral artery disease or current or previous medication.
This study was designed as an exploratory repeated, randomized, controlled crossover study. With personal devices provided by the investigators (blood pressure cuff and fingerclip), the study participants self-monitored blood pressure, heart rate, and pulse wave velocity (PWV) over 12 h during the day on 8 days (Figure 1). Measurements were taken immediately before and every 30 min over the first 3 h after consumption of capsules and at hourly intervals for a total of 12 h.
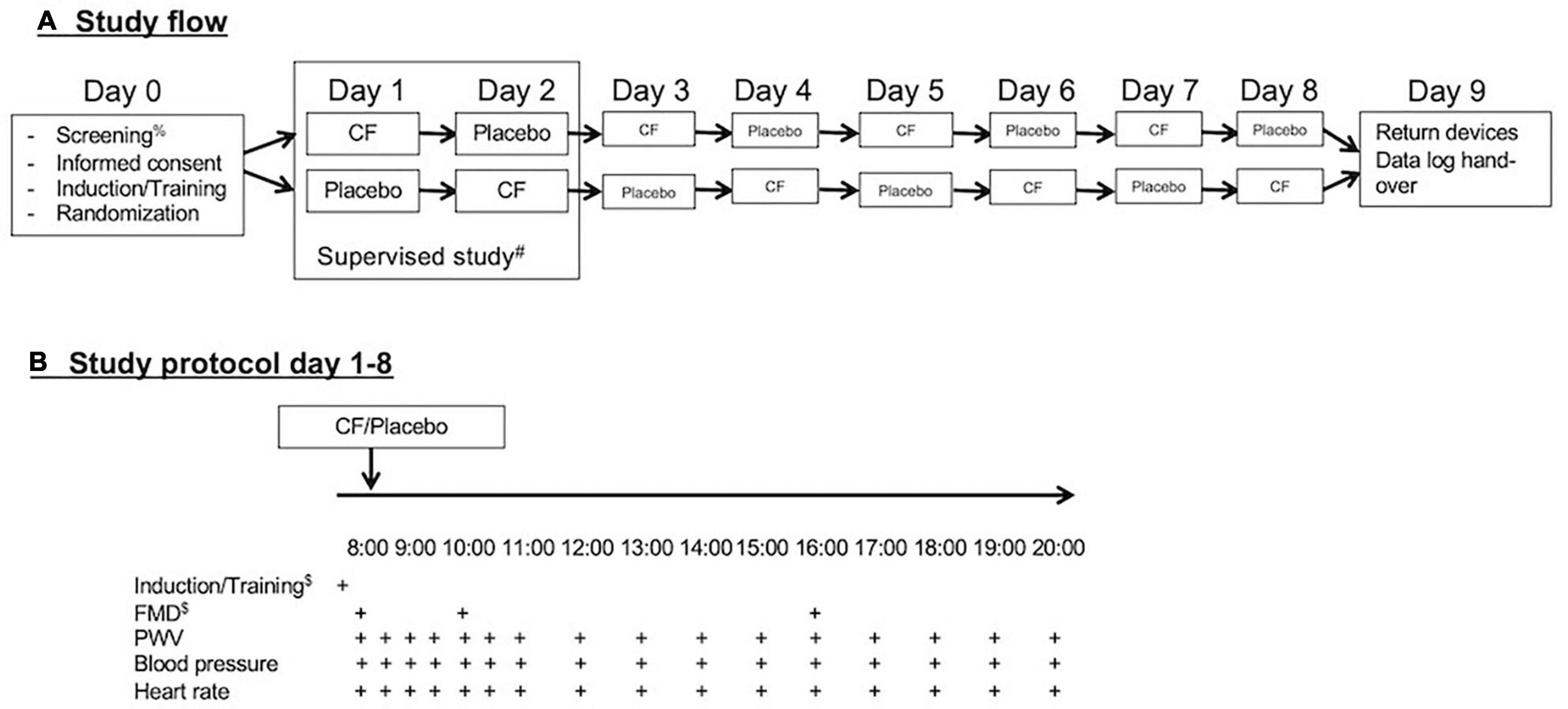
Figure 1. (A) Study flow and (B) study protocol. After screening for eligibility and obtaining signed informed consent on day 0, participants received training and were randomized. They performed the first two study days under supervision and the remaining 6 days independently via self-monitoring with personal devices during everyday life. After completion of the eight study days, the participants return their devices and submitted records of reading.
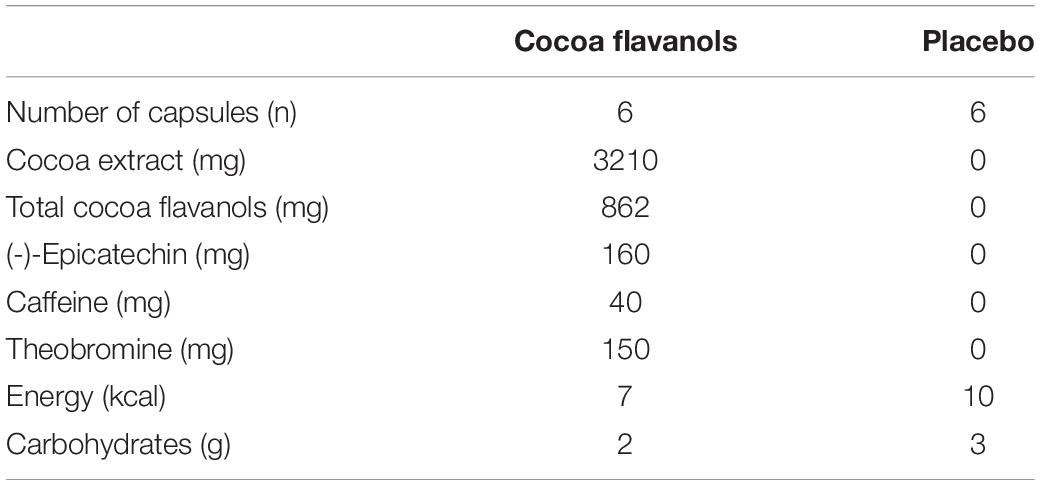
On each of the 8 study days, participants consumed either 6 cocoa flavanol capsules (containing a total of 862 mg cocoa flavanols) or 6 placebo (empty 00 capsules filled with brown sugar). All participants were randomized to one of the two different sequences of treatment periods. The alternating treatment allocations were either flavanol-placebo-flavanol-placebo-flavanol-placebo-flavanol-placebo or placebo-flavanol-placebo-flavanol-placebo-flavanol-placebo-flavanol. All capsules were provided in black opaque zip-lock bags labeled with an alphanumeric code, and capsules were supposed to be directly tipped into the mouth and swallowed with water together with breakfast.
On the first 2 days of the study, measurements were performed under supervision at the universities research facility to provide training to participants. On the following days, the measurements were performed during the normal life of the participants. The participants were instructed to sit down for 10 min before measurements and take measurements of blood pressure, heart rate, and PWV on the left arm with the arm resting on a table in front of them. They were also taught how to use the software to operate the devices on a provided small tablet device (iPod touch, Apple, United States) and introduced to an electronic case report file to which participants transferred their data.
On the training days of 8 subjects, we also performed measurements of flow-mediated dilation (FMD) at baseline before (0 h), at 2 h, and at 8 h after the first intervention to ensure the biological activity of test materials. The allocation was double-blinded and randomized, and the randomization sequence was generated using an online calculator1. The study protocol was reviewed and approved by the University of Surrey Ethics Committee with the registration number FER-1819-040, and written informed consent was obtained from all participants.
Cocoa Interventions and Placebo
The cocoa supplement and placebo capsules were purchased from Amazon.co.uk. According to the provided supplement facts, 6 capsules of CocoaVia supplement (Mars Inc., United States) contained 862 mg (95%CI: 849, 875 mg) CFs. CFs as defined here correspond to the sum of flavanol monomers and procyanidins with a degree of polymerization up to 7, according to the AOAC 2020.05 method (14). While cocoa flavanols in the supplement were initially quantified with a previously validated method, conversion to current AOAC2020.05 method reporting standards was done using a published conversion method (15). Importantly, reporting of (-)-epicatechin content remained unaffected when applying this conversion method. In addition, actual CF and (-)-epicatechin content in the supplement remained unchanged throughout the trial (15, 16). Placebo capsules were identical size empty capsules (YourSupplement Gelatin Capsules, Size 00, United Kingdom) that were filled with brown sugar. The composition is detailed in Table 1. As described earlier, capsules were consumed after baseline measurements in the morning together with breakfast. All capsules were provided to participants in black non-transparent zip-lock bags.
Measurement of Blood Pressure, Heart Rate, and Pulse Wave Velocity
Standard upper arm systolic and diastolic blood pressure and heart rate (OMRON Evolv, Milton Keynes, United Kingdom) and finger PWV (iHeart device, VitalSines International, Vancouver, BC, Canada) were measured in 30 min intervals for the first 3 h after the morning intake of the capsules and at hourly intervals over a total of 12 h (see Figure 1). The cuff size was tested during training sessions and was appropriate for all participants. The participants were asked to rest in a sitting position for 5 min prior to seated measurements with the non-dominant arm resting at heart level. Each measurement was to be performed in triplicate. The first measurement was discarded, and the average of the two remaining measurements was used for statistical analyses. The values were recorded on respective applications on an iPod touch and Excel spreadsheets both provided to participants. Participants were also instructed to refrain from taking other dietary supplements, perform exercise training, and avoid alcohol (>1 unit) on the 1 day prior to study days and the study days. The average deviation between the 2 measurements was for SBP −0.6 mmHg (range: −2.3 to 0.9 mmHg), for DBP 0.7 mmHg (range: −0.7 to 1.8 mmHg), for heart rate −1.9/min (range: −4.8 to 0.5/min), and PWV −0.1 m/s (range: −1.0 to 0.3 mmHg). The standard deviations of average deviations differed between study participants. The ranges for SDs were (SBP: 3–6 mmHg, DBP: 2–7 mmHg, heart rate: 2–5/min, and PWV: 0.2–1.0 m/s).
Flow-Mediated Dilation
All of our previous studies have confirmed positive responses to cocoa flavanols on FMD as primary end point, while only some but not others showed significant effects on blood pressure (8, 9, 17). However, FMD is difficult to measure during real life. Therefore, and to ensure that the interventions possess biological activity, we measured FMD on participants only on the first 2 days during the supervised training. Due to the alternating allocation of CF and placebo, each participant received measurements at baseline before (0 h), at 2 h, and at 8 h after the first CF and first placebo intervention. FMD measurements were only introduced after 3 people had already completed their n-of-1 trial as it became clear that the variability of blood pressure responses was greater than expected.
The FMD was measured as previously described (18). Briefly, the diameter and flow velocity of the brachial artery (BA) were measured using a 12 MHz transducer (Vivid I, GE) and automatic edge-detection software (Brachial Analyzer, Medical Imaging Applications, Iowa City, IA, United States) yielding standard deviations of mean differences between repeated measurements of less than 1%. BA diameter was measured at 2 cm proximal to the elbow. Reactive hyperemia was induced by 5 min of lower arm occlusion with a sphygmomanometer cuff inflated to 200 mmHg. Images were recorded continuously (Vascular Imager, Medical Imaging Applications, Iowa City, IA, United States) over 10 s for baseline, over within the last 30 s of occlusion (10 s recording), and over 3 min after cuff deflation. FMD was calculated as the maximal relative diameter gain relative to baseline. The FMD was expressed as (diametermax-diameterbaseline)/diameterbaseline × 100.
Statistical Analyses
Characteristics of the study population are presented as mean and standard deviation. For each parameter [systolic and diastolic blood pressure (SBP and DBP), heart rate (HR), and pulse wave velocity (PWV)], a fitted model was applied that adjusts for the baseline measure of the parameters on each study day and includes an intervention effect, time, and an interaction between time and intervention to allow for the effect to change with time. The analysis was performed first with all values over 12 h included and then repeated on a restricted dataset only including baseline and 1.5 h values. The statistical models were separately fitted for the group and for individuals (n = 1). A random subject effect accommodates the correlation between the repeated measurements in day/time within-subjects and is substituted by a random day effect in the individual models. By using the intraclass correlation coefficient (ICC), a ratio of the within-individual variance to the total, the inter-individual reliability of a measure can be indicated. High ICCs indicate that variation in measures is attributable to differences between individuals and is not dominated by day-to-day variation within individuals [see, for example, (19)].
Results
The characteristics of the study population are summarized in Table 2. The supplement was well tolerated by 10 of the participants. One participant discontinued after 5 days (3 CF, 2 placebo) due to problems in swallowing the capsules and upset stomach, but all observations of n = 11 contribute to the mixed model analysis.
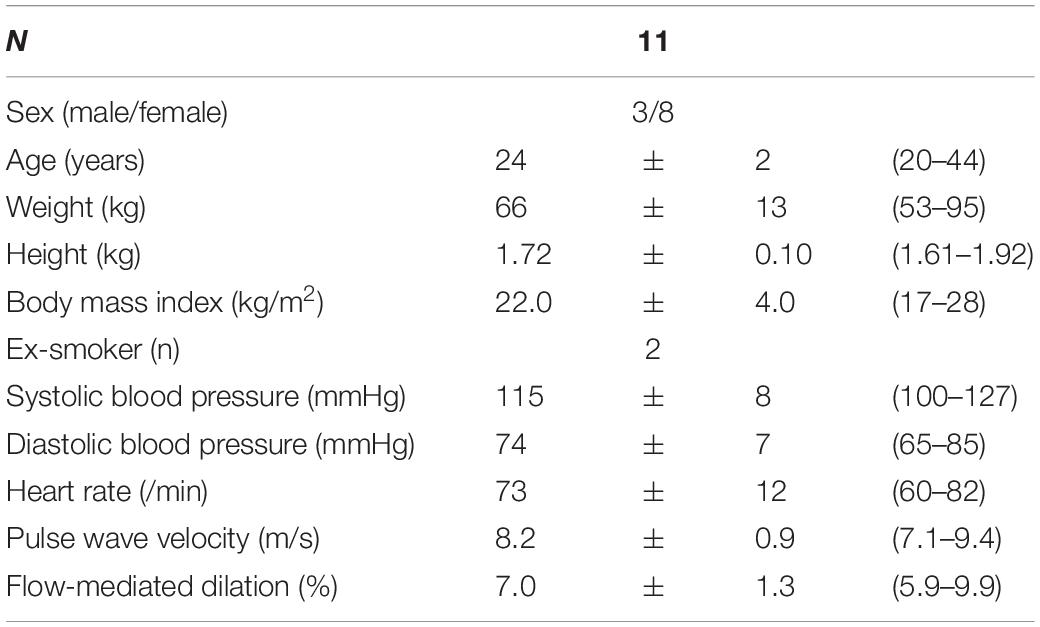
Table 2. Baseline characteristics of the study population (values are mean and standard deviation and range).
The ingestion of CF supplement but not placebo resulted in a significant increase of FMD values compared with baseline and placebo at 2 and 8 h (see Figure 2) confirming the biological activity of the used CF supplement. Supplementary Figure 1 shows the CF-related responses of the individual participants.
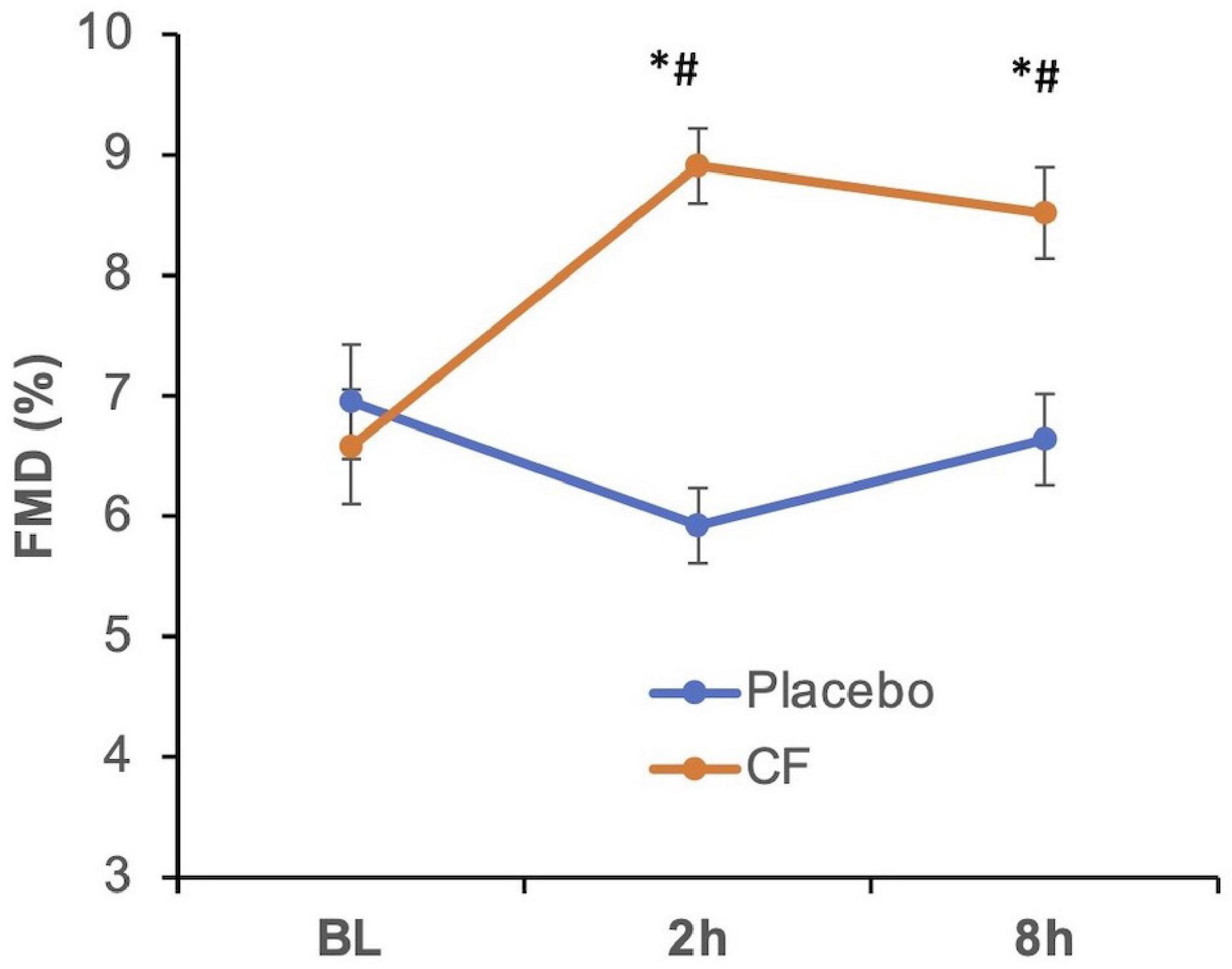
Figure 2. Effect of cocoa flavanols (CF, orange line) as compared with placebo (blue) on flow-mediated dilation (FMD) in healthy participants at baseline (BL) before cocoa flavanol or placebo (blue line) ingestion and at 2 and 8 h thereafter. Overall, p < 0.05 (two-way repeated measurements ANOVA), *p < 0.05 vs. BL, #p < 0.05 vs. respective placebo timepoint.
Analysis of Overall Effects
A first step was to evaluate the overall effects (SBP, DBP, HR, PWV) in a way that would allow comparison with other conventional studies in which participants’ measurements are taken only at selected timepoints in each study arm once for each intervention and placebo.
Figure 3 shows the averaged timecourse of blood pressure, heart rate, and PWV over 12 h. Supplementary Figure 2 shows exemplary original raw data (SBP, DBP, HR, PWV) from one of the participants. The overall mixed model analysis integrating all data showed that CF significantly decreased systolic blood pressure and PWV by −1.4 ± 0.3 mmHg (mean ± SE; pintervention < 0.0001, ptime < 0.001, pinteraction intervention*time = 0.268) and −0.11 ± 0.03 m/s (pintervention = ≤ 0.0001, ptime = 0.019, pinteraction = 0.131), respectively. There was also a decrease in diastolic blood pressure that depended on time (−0.5 ± 0.3 mmHg) (pintervention = 0.101, ptime = 0.607, pinteraction = 0.027). The heart rate significantly increased by 0.9 ± 0.4/min (p < 0.034, ptime = 0.859, pinteraction = 0.850). The responses greatly varied between participants as indicated by intra-cluster correlation coefficients (ICC) (0.41, 0.24, 0.39, 0.41). The variation in responses did not differ between the first 2 supervised and the remaining 6 home-based unsupervised measurements. Using the consecutive study days as a continuous covariate, we found no evidence that over the course of 8 days, there was a cumulative effect of CF despite interspersed placebo days.
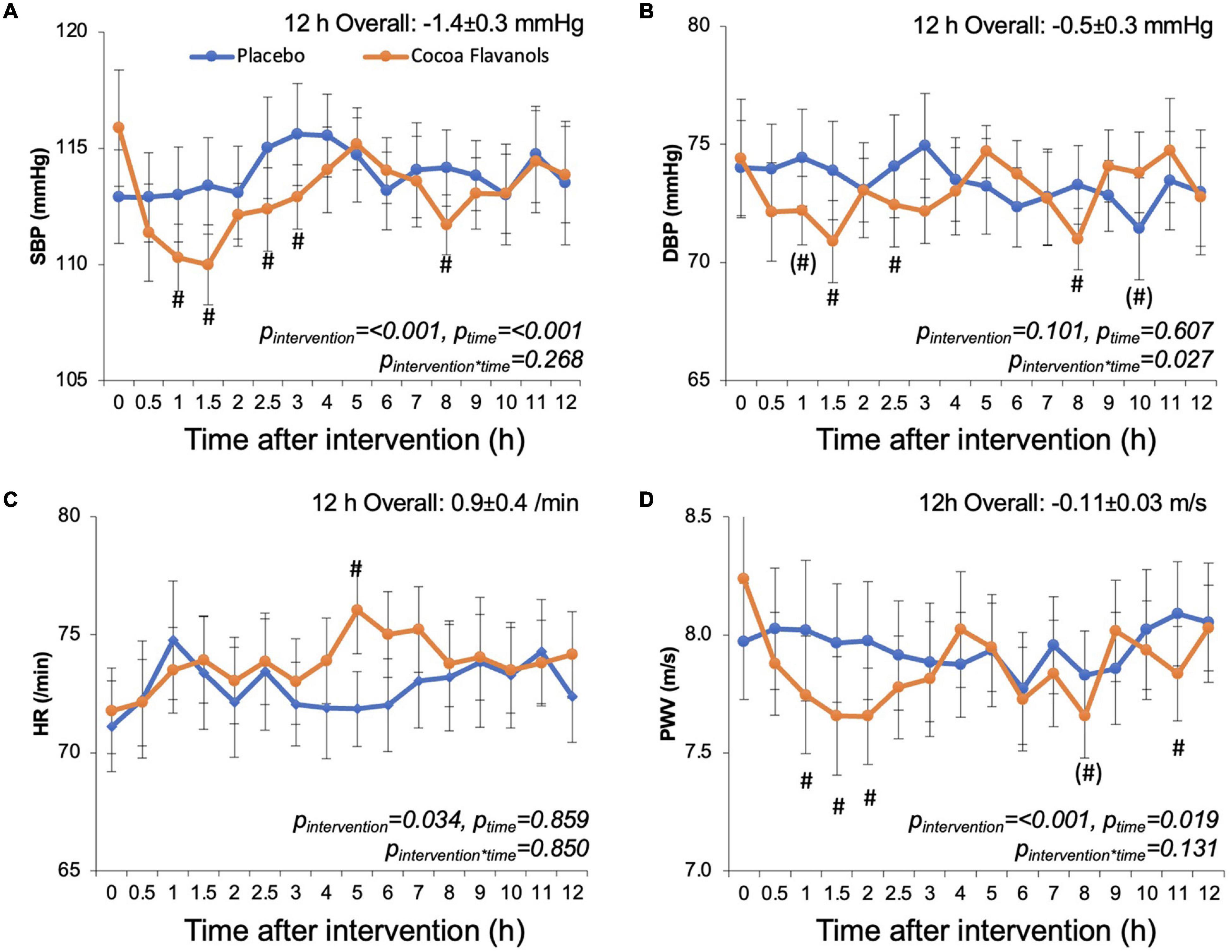
Figure 3. Effect of cocoa flavanols (orange) or placebo (blue) on time courses of (A) systolic blood pressure (SBP), (B) diastolic blood pressure (DBP), (C) heart rate (HR), and (D) pulse wave velocity (PWV). Cocoa flavanols and placebo capsules were ingested after 0 h baseline measurements. Values are average values and standard error from the average values obtained over 12 h in each of the n = 11 on the 4 cocoa flavanol days and 4 placebo days. p-values are based on a statistical mixed model adjusting for baseline measure, including intervention time and intervention × time interaction effects, #p < 0.05 and (#) p < 0.1 vs. respective placebo timepoint.
The time courses (Figure 3) were qualitatively similar in terms of SBP, DBP, and PWV with significant CF-related decreases within the first 3 h. While values returned to baseline, we observed a second somewhat smaller phase of CF-related SBP, DBP, and PWV decreases at around 8 h. The overall time course of changes in heart rate indicated a peak at around 5 h.
We then evaluated the magnitude and inter-individual variation of peak effects at 1.5 h after ingestion. This analysis showed greater effects as compared with the overall 12 h effects. SBP and PWV decreased by −4.9 ± 2.5 mmHg (pintervention = 0.032, pday = 0.645, pinteraction = 0.702). In this restricted dataset, the decrease in PWV did not reach statistical significance (−0.31 ± 1.7 m/s, pintervention = 0.060, pday = 0.269, pinteraction = 0.374). A numerical decrease in DBP was observed that was not significant (−2.4 ± 1.6 mmHg, pintervention = 0.139, pday = 0.840, pinteraction = 0.768). There was a significant time-dependent decrease in heart rate −0.5 ± 1.5/min (pintervention = 0.745, pday = 0.041, pinteraction = 0.065). The responses on the level of SBP, DBP, and PWV at 1.5 h much less varied between participants as compared with 12 h effects as indicated by low ICC (0.01, 0.10, 0.11), while ICC of heart rate was 0.59 rather indicating large between-subject variation.
Inter- and Intra-Individual Variation of Blood Pressure, Heart Rate, and Pulse Wave Velocity Responses
To further evaluate the variability of responses, we fitted statistical models to each of the 11 subjects’ dataset individually. Figure 4 shows the 12 h overall and peak 1.5 h CF responses over placebo of each participant in waterfall diagrams ordered by the magnitude of effects. The magnitude of responses greatly differed between individuals with some showing clinically quite relevant decreases of blood pressure in the order of –5 to 10 mmHg and some even showing unexpected inverse responses with statistically significant blood pressure increases in particular when evaluating the 1.5 h responses.
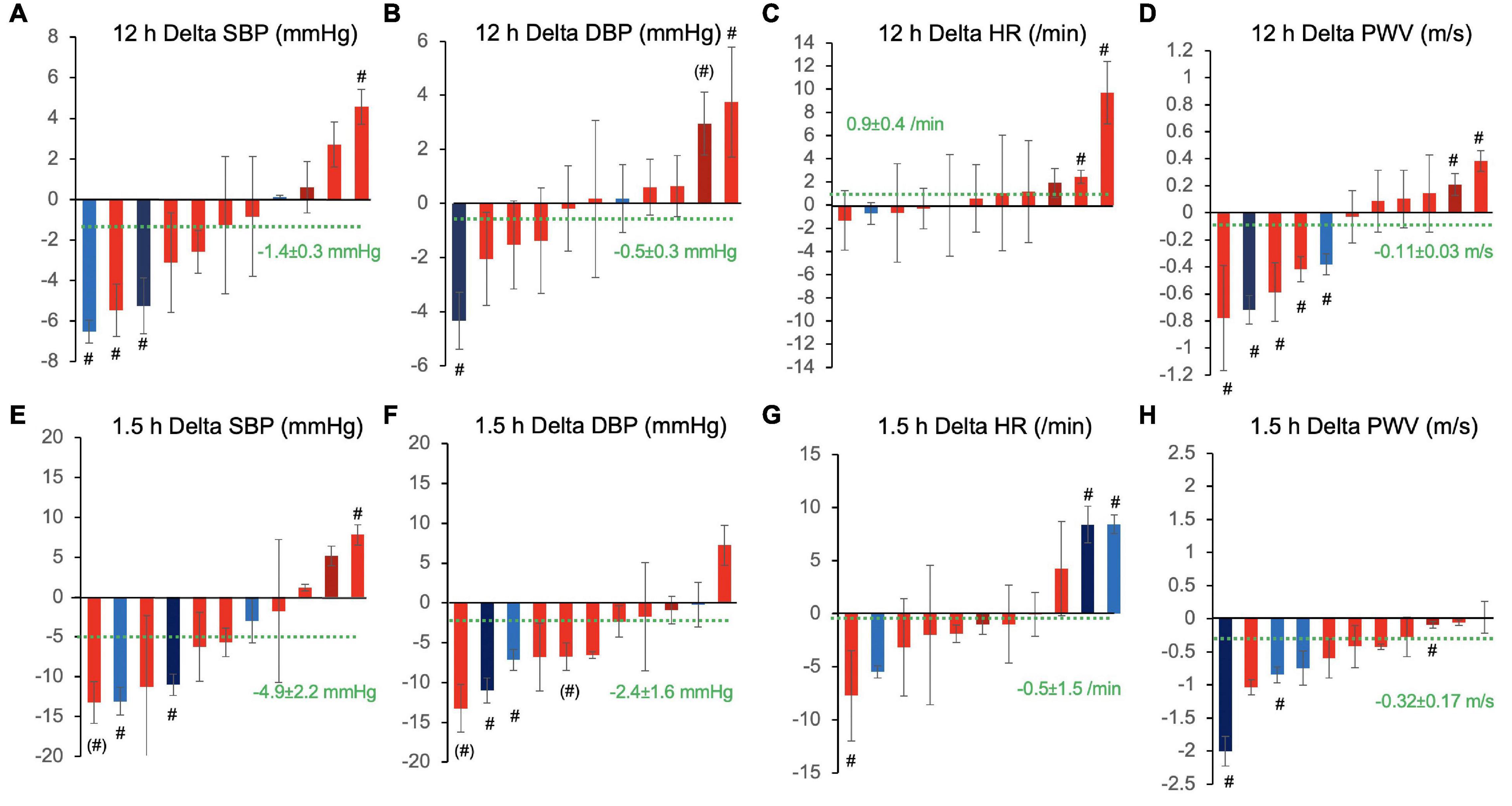
Figure 4. Inter-individual variation of 12 h overall (A–D) and 1.5 h peak responses (E–H) to cocoa flavanols as compared with placebo in terms of (A,E) systolic blood pressure (SBP), (B,F) diastolic blood pressure (DBP), (C,G) heart rate (HR), and (D,H) pulse wave velocity (PWV). Waterfall diagrams showing individual average effect in each of the 11 study participants sorted by magnitude of effects. Columns are mean effects and standard error. Green dotted line indicates the overall effect including all individuals (n = 11). #p < 0.05 and (#) p < 0.1, individual effects of cocoa flavanol intervention vs. placebo. Red shade designates female participants, blue shade designates male participants, and darker shade indicates ex-smokers.
In an attempt to identify determinants of inter-individual variability, we tested if significant correlations existed between the overall effect of CF on SBP, DBP, HR, and PWV and characteristics at baseline. There were no significant correlations between age, height, weight, BMI and FMD. Interestingly, the CF-related change in SBP, DBP, and PWV inversely correlated with baseline SBP and DBP (Figure 5). The change in heart rate was inversely correlated with baseline SBP.
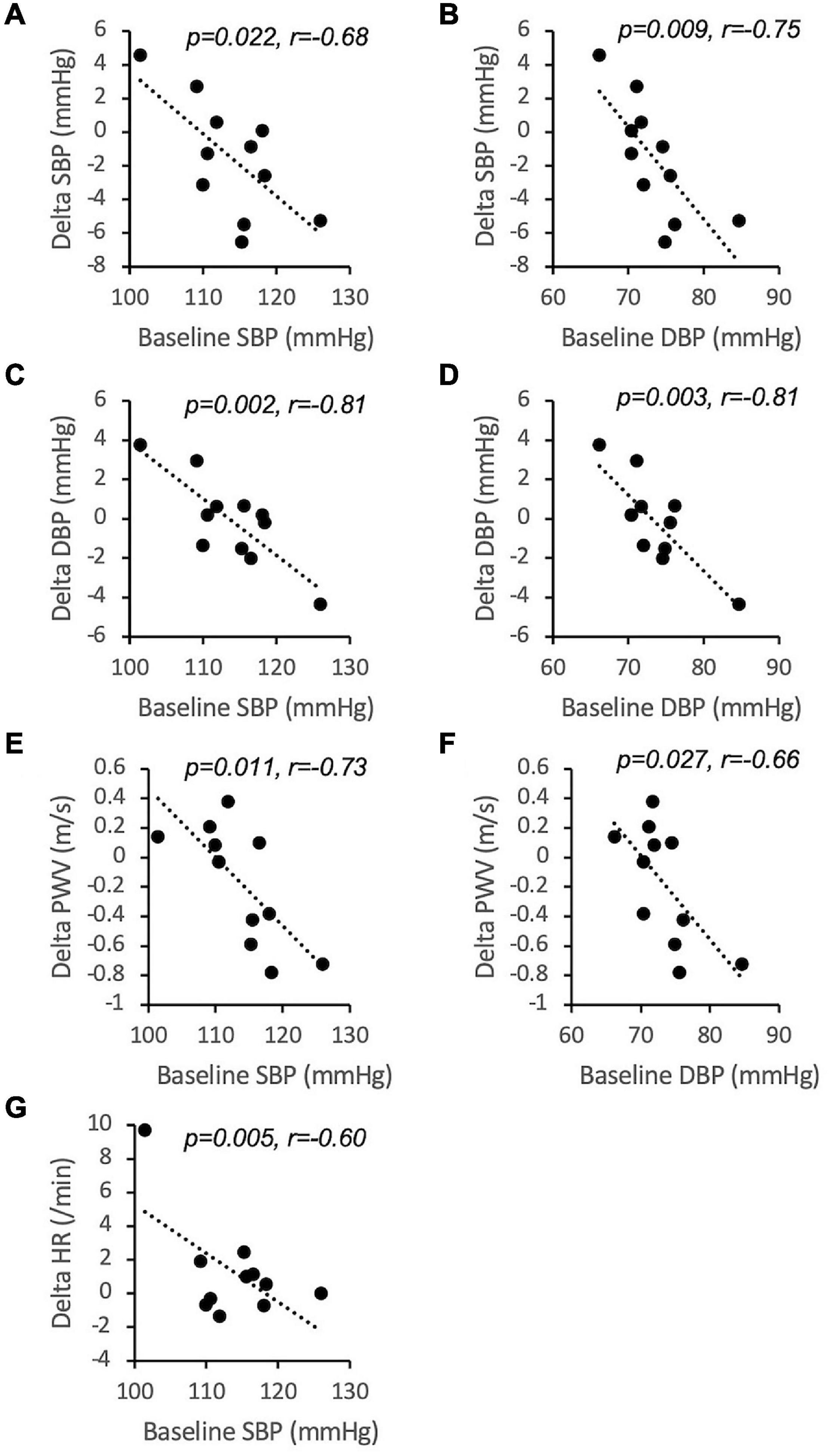
Figure 5. Inverse correlations between baseline values of systolic blood pressure (SBP, A,C,E,G) and diastolic blood pressure (DBP, B,D,F) and 12 h overall inter-individual responses to cocoa flavanols over placebo (n = 11). r is Pearson’s correlation coefficient. PWV, pulse wave velocity; HR, heart rate.
The 12 h response to CF varied also between days within participants, and the degree of variation differed between participants as indicated by wide ranges of individual ICC value (SBP: 0–0.30; DBP: 0–0.22; HR: 0–0.67; PWV: 0–0.45).
To illustrate this, Figure 6 shows baseline SBP and before and overall change of SBP after CF ingestion over 12 h (4 data points from n = 10 and 3 data points from n = 1). Note that all 11 show a linear fit line with negative slopes and that several participants showed both positive and negative responses depending on the baseline value in the morning before CF ingestion. This indicates that CF did not lower blood pressure when values were already low (approximately below 115/75 mmHg).

Figure 6. Dependence of intra-individual systolic blood pressure (SBP) responses on baseline SBP before CF intervention. Four individual CF 12 h overall responses of each of the n = 11 participants (each color represents one participant).
Discussion
This study shows for the first time a significant real-life biphasic response in terms of blood pressure and arterial stiffness lowering with maximal effects within 3 h and later at approximately 8 h. This was paralleled by increases in FMD at 2 and 8 h. The improvement of FMD by approximately 2% at 2 h was similar to what was obtained in other previous studies in healthy volunteers using slightly different cocoa flavanol interventions (9, 20, 21). The time-dependent effect of cocoa flavanols has been studied many times over the first 5–6 h after single acute ingestion and after daily consumption over days and weeks. Most of the acute studies show that peak FMD improvements occur at 1–2 h after single ingestion and return to baseline after 3 h, and daily consumption over weeks leads to a chronic improvement of FMD that lasts at least for 24 h after the last ingestion (9, 20–24). The significant increase in FMD (and decrease in blood pressure and PWV) at 8 h observed in this study has not been described previously and has interesting implications. Most studies that have performed kinetic examinations of acute single ingestion FMD effects were up to 6 h only. In these studies, FMD improved over the first 3 h with a peak effect at 1–2 h and returned to baseline by 5–6 h (17, 22). These effects may be linked to the absorption and bioavailability of structurally related epicatechin metabolites (23). However, newer literature shows that at later time points, a large proportion of cocoa flavanols that are not absorbed in the upper intestine will undergo metabolism by large intestinal microbiota that occur later in blood (25, 26). The fact that no blood samples were taken is a limitation of this study as the analysis of plasma flavanol metabolites could have provided correlative evidence in support of the gut metabolites hypothesis.
Alongside the overall analysis of the results, this study investigates for the first time intra- and inter-individual responses to cocoa flavanols using the n-of-1 study design. It shows that a large proportion of intra- and inter-individual variation effects were related to baseline blood pressure values. Not all participants responded and not all responded all the time. Specifically, if the blood pressure was low in a person on a certain day, the cocoa flavanols decreased blood pressure and arterial stiffness less. This has important safety and mechanistic implications. In particular, in primary prevention, safety is a major issue, and excessive blood pressure lowering may pose a safety risk as described for antihypertensive medication (27) for the potential use of flavanols in primary prevention in low-risk individuals without risk factors and normal blood pressure (28). The magnitude of effects in particular within the first 3 h is similar to what standard antihypertensive medications achieve in clinical trials (29), highlighting the clinical relevance and potential of flavanols for use in clinical practice. Whether or not a continuous decrease in blood pressure can be achieved with repetitive administration remains to be investigated. The increase in heart rate we have observed at around 5 h may be due to the high theobromine content of the cocoa supplement and needs to be taken into account as a potential side effect (30) that could also mask blood pressure lowering effects of CF. In particular in young and healthy people, an increase in heart rate is a physiological response to peripheral vasodilation and can even prevent blood pressure lowering of antihypertensive drugs like calcium channel blockers (amlodipine) (31). This is also why calcium channel blockers exert more effective blood pressure lowering when combined with beta blockers that prevent compensatory increase in heart rate (32). In line with our findings, the results from previous studies investigating blood pressure effects of cocoa flavanols were less consistent than studies investigating FMD (33). The mechanisms of how cocoa flavanols improve vascular function are not entirely understood beyond the knowledge that nitric oxide bioavailability is likely causally involved in acute effects (23), and long-term effects, which are less comparable with this study, are linked to changes in gene expression profiles (34). Whether the same mechanisms mediate effects on endothelial function, blood pressure, and arterial stiffness is not known. Of note, gene expression changes that correlated with changes in FMD significantly differed from those associated with blood pressure and PWV changes (34). One may argue that this study supports that cocoa flavanols rather improve vascular homeostasis than being direct vasoactive molecules as the latter would be expected to lower blood pressure more and even if low at baseline.
The variability of responses highlights the importance of personal devices that can monitor biomarkers of biological effects frequently as therapeutic targets for personalized medicine. For instance, home blood pressure monitoring is now recommended in clinical practice guidelines (28). Our data clearly demonstrate that people respond differently to identical treatments and even one person may respond differently on different days. Wearable devices to measure the responses frequently or even continuously will be an important tool to understand individual responses and gauge individual treatments effectively. The devices used in this study required manual activation of measurements that limits wider implementation. The development of automated non-invasive devices for measuring blood pressure (35) without the need for a cuff inflation will be important to avoid interruption of normal life or sleep.
The effective exploitation of n-of-1 trials for personalized medicine requires standardized statistical models to analyze the results, and there is currently no standard approach. In this study, we adopted mixed model approaches that allow adjustments for baseline measures of the parameters on each day and include an intervention effect, time, and an interaction between time and intervention, as well as random subject or day effects to appropriately capture the between- and within-subject variation. The ability to compare the pooled analysis over subjects and study individual effects demonstrates the importance of further investigation of individualized approaches over simple population-based methods.
Several limitations not previously mentioned apply to this study. The small sample size limits generalizability to the wider population. Furthermore, the effect of CFs may be influenced by the diet and, in particular, the breakfast with which it was consumed. Unfortunately, we did not collect data related to the diet consumed throughout the study or take blood samples to evaluate biomarkers of polyphenol intake to investigate this. Another limitation relates to the fact that the cocoa supplement did not only contain cocoa flavanols but also significant amounts of methylxanthines, primarily theobromine. It is now appreciated that methylxanthines can have health effects on their own (36). The placebo in this study did not contain any methylxanthines. However, we have previously demonstrated that methylxanthines at up to 200 mg do not exert significant effects on endothelial function, blood pressure, and arterial stiffness in healthy young volunteers but rather increase the bioavailability of flavanols over the first 5 h (21). Therefore, it is reasonable to assume that most effects with the exception of heart rate in this study are driven by the flavanols. In addition, we did not test endothelium-independent vasodilation in response to nitroglycerin which is often part of the vascular assessment protocol (37). Therefore, endothelium independent effects cannot be excluded, but are rather unlikely as a number of previous studies did not show any effects on nitroglycerin-mediated dilation in healthy populations (8, 9). Finally, we need to point out that effects on PWV are unlikely to be due to short-term effects on arterial structural stiffness but are most likely secondary to blood pressure lowering effects. Finally, the amounts of CF [862 mg total, 160 mg (-)-epicatechin] applied in this study are relatively high but are in the range that can be achieved with regular diet. The mean daily intake of total flavanols in central Europe was estimated based on questionnaires at 449 mg [13–14 mg (-)-epicatechin] (38, 39). A more recent study indicates a steep dose response between all-cause, cardiovascular and cancer mortality, and flavanol monomer intake up to approximately 100 mg/day and then plateaus (2). The daily flavanol monomer intake reported in this study ranged from 0 to 916 mg (oligo + polymers: 0–2,254 mg) (2). The dose to achieve half maximal effects in humans (ED50) in terms of acute endothelial functional increases at 2 h after single ingestion is approximately 0.5 mg (-)-epicatechin/kg [body weight] in healthy humans with maximal effects achieved at ca. 2 mg/kg (21, 40). The dose response in terms of blood pressure decreases is not well established but may require higher amounts (41). Therefore, the amounts administered were chosen as they are in the range that can be achieved with normal diet and would be expected to maximally improve endothelial function. They are similar to what was used in the recent COSMOS trial (500 mg CF/day) that showed a significant reduction in cardiovascular mortality when consumed over several years (42).
In conclusion, our data confirm that cocoa can improve vascular function and decrease blood pressure and arterial stiffness not only within the first 3 h after ingestion but also later at 8 h in healthy normotensive people. Our study uncovers considerable intra- and inter-individual variation in responses using an innovative n-of-1 study design with personal devices. With this approach, we uncovered baseline blood pressure as a major determinant of response in healthy young people. Our data highlight the need for personal health monitors to develop and implement effective personalized nutritional intervention strategies in the future.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the University of Surrey Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MB: project administration and preparation, investigation, analysis, and writing – review and editing. PC: supervision and review and editing. AR-M: review and editing. SS: statistical analysis and review and editing. CH: conceptualization, project administration, supervision, investigation, analysis, and writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
MB was funded by an institutional grant of Health Education England to CH. This study was funded by the University of Surrey as part of the Biomedical Science final year Bachelor of Science research project (BMS3049). CH and SS have received funding from the Economic and Social Research Council (ES/V00980X/1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.886597/full#supplementary-material
Footnotes
References
1. Zhong GC, Hu TY, Yang PF, Peng Y, Wu JJ, Sun WP, et al. Chocolate consumption and all-cause and cause-specific mortality in a US population: a post hoc analysis of the PLCO cancer screening trial. Aging (Albany NY). (2021) 13:18564–85. doi: 10.18632/aging.203302
2. Bondonno NP, Dalgaard F, Kyro C, Murray K, Bondonno CP, Lewis JR, et al. Flavonoid intake is associated with lower mortality in the Danish diet cancer and health cohort. Nat Commun. (2019) 10:3651. doi: 10.1038/s41467-019-11622-x
3. Bondonno NP, Lewis JR, Blekkenhorst LC, Bondonno CP, Shin JH, Croft KD, et al. Association of flavonoids and flavonoid-rich foods with all-cause mortality: the blue mountains eye study. Clin Nutr. (2020) 39:141–50. doi: 10.1016/j.clnu.2019.01.004
4. Dalgaard F, Bondonno NP, Murray K, Bondonno CP, Lewis JR, Croft KD, et al. Associations between habitual flavonoid intake and hospital admissions for atherosclerotic cardiovascular disease: a prospective cohort study. Lancet Planet Health. (2019) 3:e450–9. doi: 10.1016/S2542-5196(19)30212-8
5. Gopinath B, Liew G, Lewis JR, Bondonno NP, Bondonno CP, Burlutsky G, et al. Associations between dietary flavonoids and retinal microvasculature in older adults. Eur J Nutr. (2019) 59:3093–3101.
6. Parmenter BH, Croft KD, Hodgson JM, Dalgaard F, Bondonno CP, Lewis JR, et al. An overview and update on the epidemiology of flavonoid intake and cardiovascular disease risk. Food Funct. (2020) 11:6777–806. doi: 10.1039/d0fo01118e
7. Heiss C, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. Eur Heart J. (2010) 31:2583–92. doi: 10.1093/eurheartj/ehq332
8. Heiss C, Sansone R, Karimi H, Krabbe M, Schuler D, Rodriguez-Mateos A, et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial. Age (Dordr). (2015) 37:9794. doi: 10.1007/s11357-015-9794-9
9. Sansone R, Rodriguez-Mateos A, Heuel J, Falk D, Schuler D, Wagstaff R, et al. Cocoa flavanol intake improves endothelial function and Framingham risk score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola health study. Br J Nutr. (2015) 114:1246–55. doi: 10.1017/S0007114515002822
10. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. (2017) 4:CD008893.
11. Vohra S, Shamseer L, Sampson M, Bukutu C, Schmid CH, Tate R, et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015 Statement. J Clin Epidemiol. (2016) 76:9–17. doi: 10.1136/bmj.h1738
12. Gabler NB, Duan N, Vohra S, Kravitz RL. N-of-1 trials in the medical literature: a systematic review. Med Care. (2011) 49:761–8. doi: 10.1097/MLR.0b013e318215d90d
13. Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. (2011) 8:161–73. doi: 10.2217/pme.11.7
14. Bussy U, You H, Kwik-Uribe C. Determination of cocoa flavanols and procyanidins (by degree of polymerization DP1-7) in cocoa-based products by hydrophilic interaction chromatography coupled with fluorescence detection, collaborative study. J AOAC Int. (2022). doi: 10.1093/jaoacint/qsac007
PubMed Abstract | CrossRef Full Text [Epub ahead of print]. | Google Scholar
15. Bussy U, Ottaviani JI, Kwik-Uribe C. Evolution of cocoa flavanol analytics: impact on reporting and cross-study comparison. Food Funct. (2021) 12:3433–42. doi: 10.1039/d1fo00215e
16. Bussy U, Hewitt G, Olanrewaju Y, May BR, Anderson N, Ottaviani JI, et al. Single-laboratory validation for the determination of cocoa flavanols and procyanidins (by degree of polymerization DP1-7) in cocoa-based products by hydrophilic interaction chromatography coupled with fluorescence detection: first action 2020.05. J AOAC Int. (2021) 104:413–21. doi: 10.1093/jaoacint/qsaa132
17. Heiss C, Kleinbongard P, Dejam A, PerrÇ S, Schroeter H, Sies H, et al. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. (2005) 46:1276–83. doi: 10.1016/j.jacc.2005.06.055
18. Rodriguez-Mateos A, Hezel M, Aydin H, Kelm M, Lundberg JO, Weitzberg E, et al. Interactions between cocoa flavanols and inorganic nitrate: additive effects on endothelial function at achievable dietary amounts. Free Radic Biol Med. (2014) 80:121–8. doi: 10.1016/j.freeradbiomed.2014.12.009
19. Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. (2005) 30:698–714. doi: 10.1016/j.psyneuen.2005.03.002
20. Rodriguez-Mateos A, Weber T, Skene SS, Ottaviani JI, Crozier A, Kelm M, et al. Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: randomized, controlled, double-masked intervention trial. Am J Clin Nutr. (2018) 108:1229–37. doi: 10.1093/ajcn/nqy229
21. Sansone R, Ottaviani JI, Rodriguez-Mateos A, Heinen Y, Noske D, Spencer JP, et al. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: randomized, double-masked controlled studies. Am J Clin Nutr. (2017) 105:352–60. doi: 10.3945/ajcn.116.140046
22. Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. (2003) 290:1030–1. doi: 10.1001/jama.290.8.1030
23. Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. (2006) 103:1024–9. doi: 10.1073/pnas.0510168103
24. Heiss C, Finis D, Kleinbongard P, Hoffmann A, Rassaf T, Kelm M, et al. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol. (2007) 49:74–80. doi: 10.1097/FJC.0b013e31802d0001
25. Ottaviani JI, Borges G, Momma TY, Spencer JP, Keen CL, Crozier A, et al. The metabolome of [2-(14)C](-)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci Rep. (2016) 6:29034. doi: 10.1038/srep29034
26. Ottaviani JI, Heiss C, Spencer JPE, Kelm M, Schroeter H. Recommending flavanols and procyanidins for cardiovascular health: revisited. Mol Aspects Med. (2018) 61:63–75. doi: 10.1016/j.mam.2018.02.001
27. Albasri A, Hattle M, Koshiaris C, Dunnigan A, Paxton B, Fox SE, et al. Association between antihypertensive treatment and adverse events: systematic review and meta-analysis. BMJ. (2021) 372:n189. doi: 10.1136/bmj.n189
28. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2021) 42:3227–337.
29. Canoy D, Copland E, Nazarzadeh M, Ramakrishnan R, Pinho-Gomes AC, Salam A, et al. Antihypertensive drug effects on long-term blood pressure: an individual-level data meta-analysis of randomised clinical trials. Heart. (2022). doi: 10.1136/heartjnl-2021-320171
PubMed Abstract | CrossRef Full Text [Epub ahead of print]. | Google Scholar
30. van den Bogaard B, Draijer R, Westerhof BE, van den Meiracker AH, van Montfrans GA, van den Born B-JH. Effects on peripheral and central blood pressure of cocoa with natural or high-dose theobromine: a randomized, double-blind crossover trial. Hypertension. (2010) 56:839–46. doi: 10.1161/HYPERTENSIONAHA.110.158139
31. Stankovic S, Panz V, Klug E, Di Nicola G, Joffe BI. Amlodipine and physiological responses to brisk exercise in healthy subjects. Cardiovasc Drugs Ther. (1999) 13:513–7. doi: 10.1023/a:1007875603969
32. Hostalek-Gottwald U, Gaciong Z. A growing evidence base for the fixed-dose combination of bisoprolol and amlodipine to manage hypertension. Curr Med Res Opin. (2022) 1–5. doi: 10.1080/03007995.2022.2072087
PubMed Abstract | CrossRef Full Text [Epub ahead of print]. | Google Scholar
33. Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. (2012) 95:740–51. doi: 10.3945/ajcn.111.023457
34. Milenkovic D, Rodriguez-Mateos A, Lucosz M, Istas G, Declerck K, Sansone R, et al. Flavanol consumption in healthy men preserves integrity of immunological-endothelial barrier cell functions: nutri(epi)genomic analysis. Mol Nutr Food Res. (2022) e2100991. doi: 10.1002/mnfr.202100991
PubMed Abstract | CrossRef Full Text [Epub ahead of print].| Google Scholar
35. Hosanee M, Chan G, Welykholowa K, Cooper R, Kyriacou PA, Zheng D, et al. Cuffless single-site photoplethysmography for blood pressure monitoring. J Clin Med. (2020) 9:723. doi: 10.3390/jcm9030723
36. Kim J, Kim J, Shim J, Lee CY, Lee KW, Lee HJ. Cocoa phytochemicals: recent advances in molecular mechanisms on health. Crit Rev Food Sci Nutr. (2014) 54:1458–72. doi: 10.1080/10408398.2011.641041
37. Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. (2019) 40:2534–47. doi: 10.1093/eurheartj/ehz350
38. Vogiatzoglou A, Mulligan AA, Bhaniani A, Lentjes MA, McTaggart A, Luben RN, et al. Associations between flavan-3-ol intake and CVD risk in the Norfolk cohort of the European prospective investigation into cancer (EPIC-Norfolk). Free Radic Biol Med. (2015) 84:1–10. doi: 10.1016/j.freeradbiomed.2015.03.005
39. Vogiatzoglou A, Mulligan AA, Luben RN, Lentjes MAH, Heiss C, Kelm M, et al. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European union. Br J Nutr. (2014) 111:1463–73. doi: 10.1017/S0007114513003930
40. Rodriguez-Mateos A, Hezel M, Aydin H, Kelm M, Lundberg JO, Weitzberg E, et al. Interactions between cocoa flavanols and inorganic nitrate: additive effects on endothelial function at achievable dietary amounts. Free Radic Biol Med. (2015) 80:121–8. doi: 10.1016/j.freeradbiomed.2014.12.009
41. Davison K, Berry NM, Misan G, Coates AM, Buckley JD, Howe PR. Dose-related effects of flavanol-rich cocoa on blood pressure. J Hum Hypertens. (2010) 24:568–76. doi: 10.1038/jhh.2009.105
42. Sesso HD, Manson JE, Aragaki AK, Rist PM, Johnson LG, Friedenberg G, et al. Effect of cocoa flavanol supplementation for prevention of cardiovascular disease events: the COSMOS randomized clinical trial. Am J Clin Nutr. (2022). doi: 10.1093/ajcn/nqac055
PubMed Abstract | CrossRef Full Text [Epub ahead of print]. | Google Scholar
Keywords: cocoa flavanols, n-of-1, blood pressure, pulse wave velocity, variability, flow-mediated dilation
Citation: Bapir M, Campagnolo P, Rodriguez-Mateos A, Skene SS and Heiss C (2022) Assessing Variability in Vascular Response to Cocoa With Personal Devices: A Series of Double-Blind Randomized Crossover n-of-1 Trials. Front. Nutr. 9:886597. doi: 10.3389/fnut.2022.886597
Received: 28 February 2022; Accepted: 17 May 2022;
Published: 13 June 2022.
Edited by:
Antje R. Weseler, Hochschule für Gesundheit, GermanyReviewed by:
Sonia Ramos, Spanish National Research Council (CSIC), SpainTelmo Pereira, Fisiologia Clínica, Portugal
Elsa Heyman, Université de Lille, France
Copyright © 2022 Bapir, Campagnolo, Rodriguez-Mateos, Skene and Heiss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Heiss, c.heiss@surrey.ac.uk
 Mariam Bapir
Mariam Bapir Paola Campagnolo
Paola Campagnolo Ana Rodriguez-Mateos
Ana Rodriguez-Mateos Simon S. Skene
Simon S. Skene Christian Heiss
Christian Heiss