Recommendations for Assessment of Environmental Exposures in Longitudinal Life Course Studies Such as the National Children's Study
- 1Westat, Rockville, MD, United States
- 2Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, United States
- 3Department of Pscychology, Northwestern University, Evanston, IL, United States
- 4Department of Anthropology, Northwestern University, Evanston, IL, United States
- 5University of Washington, Seattle, WA, United States
- 6Icahn School of Medicine at Mount Sinai School, New York, NY, United States
An important step toward understanding the relationship between the environment and child health and development is the comprehensive cataloging of external environmental factors that may modify health and development over the life course. Our understanding of the environmental influences on health is growing increasingly complex. Significant key questions exist as to what genes, environment, and life stage mean to defining normal variations and altered developmental trajectories throughout the life course and also across generations. With the rapid advances in genetic technology came large-scale genomic studies to search for the genetic etiology of complex diseases. While genome-wide association studies (GWAS) have revealed genetic factors and networks that advance our understanding to some extent, it is increasingly recognized that disease causation is largely non-genetic and reflects interactions between an individual's genetic susceptibility and his or her environment. Thus, the full promise of the human genome project to prevent or treat disease and promote good health arguably depends on a commitment to understanding the interactions between our environment and our genetic makeup and requires a design with prospective environmental data collection that considers critical windows of susceptibility that likely correspond to the expression of specific genes and gene pathways. Unlike the genome, which is static, relevant exposures as well as our response to exposures, change over time. This has fostered the complementary concept of the exposome ideally defined as the measure of all exposures of an individual over a lifetime and how those exposures relate to health. The exposome framework considers multiple external exposures (e.g., chemical, social) and behaviors that may modify exposures (e.g., diet), as well as consequences of environmental exposures indexed via biomarkers of physiological response or measures of behavioral response throughout the lifespan. The exposome concept can be applied in prospective developmental studies such as the National Children's Study (NCS) with the practical understanding that even a partial characterization will bring major advances to health. Lessons learned from the NCS provide an important opportunity to inform future studies that can leverage these evolving paradigms in elucidating the role of environment on health across the life course.
Introduction
Measuring the Environment
The etiology of health and well-being is increasingly recognized to result from the complex interplay of environmental influences operating at multiple levels, including the individual, the home and family, the neighborhood/community, and beyond (1, 2). As part of this growing complexity, evidence suggests that connections between health and economic well-being are embedded within the larger context of people's lives. Health outcomes are clearly socially patterned with greater burden related to lower socioeconomic status (SES) as well as ethnic/minority group membership. Lower SES, ethnic minority group status, and residence in a more toxic environment are closely intertwined in the United States (US). Among low-SES areas, those with predominantly minority, segregated populations seem especially burdened. In addition, studies in urban environments show geographic variation in health outcomes across large cities and neighborhoods within cities that cannot be explained by economic factors alone. Although available data are more limited, studies in rural areas also suggest the stratification of risk based on SES and the proportion of minorities. The principles of social and environmental justice are thus inherently linked to health. Populations of lower socioeconomic position are disproportionately exposed to chemical stressors including irritants (e.g., tobacco smoke), pollutants (e.g., diesel-related particles), and housing-related toxicants (e.g., indoor allergens). Moreover, individuals may also live in families and communities that are differentially burdened with social toxins (e.g., crime or interpersonal violence exposure, discrimination, financial strain), which, in turn, may be related to the variable experiencing of psychosocial stress. While far more attention has been given to physical environmental toxicants, more recent consensus statements by the National Academies of Science (2) and the National Institutes of Health (3) point to the need to consider social environmental and behavioral factors as well. Indeed, non-chemical stressors (i.e., social determinants and psychological stress) may be as toxic as chemical pollutants in the environment (4).
As depicted in Figure 1, the proposed approach to assessing environmental risk in the NCS took a multi-level approach that explicitly recognized the embedding of health risk within its biologic, socioeconomic, social, and physical environmental as well as community, and societal contexts (5). Such an approach may help to explain heterogeneities in disease expression across development as well as socioeconomic and geographic boundaries. This framework also incorporated the interdisciplinary life course framework that expands on the developmental origins view and emphasizes the physical, biological, and social/behavioral factors throughout life that independently, cumulatively, and interactively influence health and development (3, 6). The conceptualization of the exposome accommodates this recognized complexity and provided the overarching organizational framework that guided recommendations for environmental assessments in the NCS. The exposome considers multiple exposures (e.g., social and physical) and behaviors that may influence exposure to environmental factors (e.g., diet, smoking, drug use, use of consumer products, parenting styles), as well as consequences of environmental exposures indexed via biomarkers (e.g., epigenetic changes and/or associated biological responses or endogenous processes such as immunomodulation or oxidative stress) and behavioral constructs (e.g., emotion regulation, temperament, attributional style) to more comprehensively characterize the impact of environmental exposures throughout the lifespan (see Figure 2).
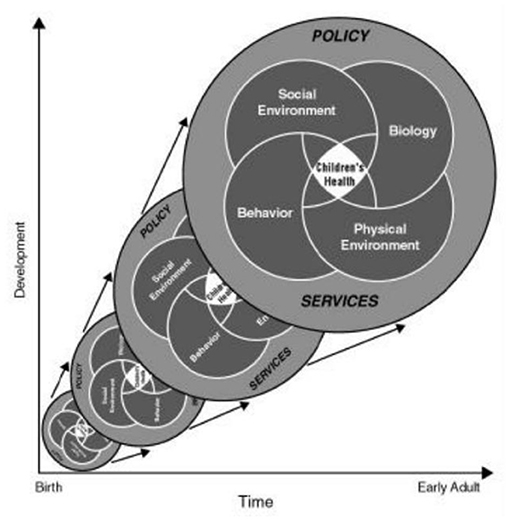
Figure 1. Characterizing environment across the life course: an ecological approach (http://www.ncbi.nlm.nih.gov/books/NBK92206/). Policy and services affect the social environment, biology, behavior, and physical environment, which in turn affect children's health. These factors change across time (x-axis) and development (y-axis), influencing children's health along the way.
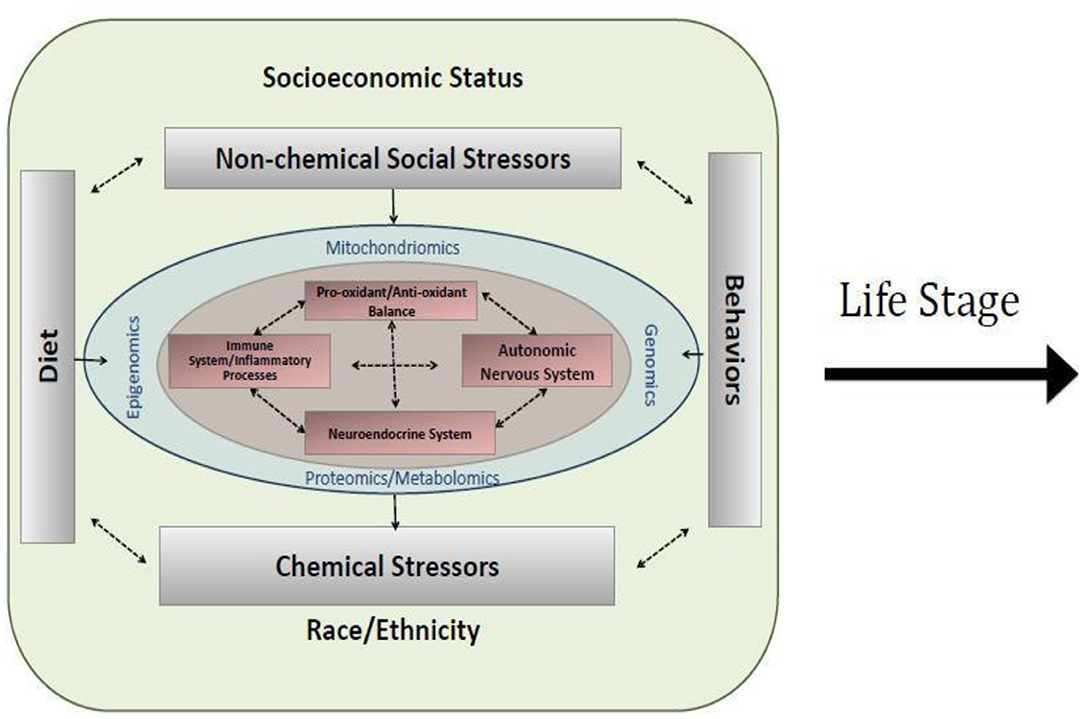
Figure 2. Conceptualization of a cross-section of the exposome within a life stage interval. Socioeconomic status, non-chemical stressors, race/ethnicity, chemical stressors, diet, and behavior interact with genomics, epigenomics, mitochondiomics, and proteomics/metabolomics to influence healthy biological functioning, including oxidant/antioxidant balance, autonomic nervous system, inflammation, and neuroendocrine functioning.
Figure 2 also depicts key regulatory systems (immune system, neuroendocrine system, autonomic nervous system, and pro-oxidant/anti- oxidant balance) that may be influenced by environmental factors and consequently program health trajectories. While mechanisms underlying programming effects of the wide array of environmental factors thought to contribute to health and development are not completely elucidated, banking biospecimens in critical windows of development to allow more comprehensive approaches to understanding networks and mechanisms that may be involved is critical. For the NCS, this included taking advantage of evolving high-throughput “omics” approaches, some of which are depicted in Figure 2 (epigenomics, metabolomics, mitochoriomics, microbiomics). For example, epigenetics, the study of changes in gene expression without change in DNA sequence, has emerged as a leading mechanism through which environment may impact health and development in a dynamic way over the life course. A growing number of studies have demonstrated associations between DNA methylation, a leading epigenetic mechanism, and exposures to a range of chemical and non-chemical environmental stressors. While much has been documented on the importance of environmentally induced epigenetic changes for programming health in early development (prenatal, early childhood) (2, 7, 8), this is an important mechanism to understand across developmental periods, for example, recent evidence suggests a role over a range of developmental windows in relation to pubertal timing (9). Microbiomics, the study of changes in the oral and gut microbiome, have been increasingly associated with metabolic changes and diseases such as cardiovascular, gastrointestinal, and pancreatic cancer risks. For example, in healthy mouths, the oral microbiome is dominated by Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Spirochaetes, and Fusobacteria; however, in oral diseases, lactobacilli and Streptococcus mutans produce excessive amounts of acid to produce dental cavities. P. catoniae and N. flavescens were associated with cavity-free mouths. Bacterial communities can be determined from biospecimens using pyrosequencing or 16SrDNA metagenomic analysis of buccal cells and fecal samples. The interplay with mithochondrial processes has emerged more recently in relation to environmental influences on health and development (10). While “omics” approaches are more extensively discussed by the Physical Health & Systems Domain, we briefly introduce these concepts here to exemplify their central importance to the concept of measuring the exposome to study environmental influences on health and development in the NCS.
Conceptually, the framework needs to account for a reasonably comprehensive spectrum of exposures, timing and duration of exposures, and acute and chronic responses to the exposures (e.g., behavioral, physiologic) over time. In a longitudinal study design, this will allow researchers to ask, “Where are impacts and responses occurring? Is it at the cellular, the genetic, or the organ, individual, household, specific population group, or community level?” Indeed, the answers are unlikely to be exclusive.
Need for a Developmental Framework
Effects to children can occur from exposures at various stages in the life course, including preconception. Effects on either the sperm or egg prior to conception have been known to impact development and, in many cases, overall viability of the conceptus. Recent emphasis on heritable epigenetic changes that occur via either germ cell has advanced not only the concept of male-mediated developmental toxicity but also illustrated a myriad of non-coding RNA mechanisms including maternal inheritance. In the male, both progenitor and replenishing spermatogonial cells have allowed the male to repair and fertility to return in some cases. However, high doses of environmental agents can also destroy male reproductive capability beyond repair. It is also becoming increasingly apparent that certain phenotypes are inherited across generations independent of the information contained in the DNA sequence, by factors in germ cells that remain largely uncharacterized (11). Cohort studies should attempt to collect information concerning the exposures to the grandparents and parents, particularly in their own childhood.
Plasticity, the ability of the organism to respond to an insult and still maintain homeostasis, can be overcome, and health impacts can arise as a consequence of environmental exposures during critical life periods affecting key physiological systems that operate in orchestrating underlying developmental processes (12). The concept that factors other than genetic susceptibility act early in life to permanently organize or imprint physiological systems is known as perinatal programming and has formed the basis of the developmental origins of disease research. The NCS pregnancy cohort will allow us to examine the implications of a number of environmental exposures in this vulnerable period of development. Prenatally, children are particularly vulnerable to disruption of developmental processes during relatively narrow time windows of development. Exposure to environmental toxicants during prenatal and/or early postnatal development may alter the normal course of morphogenesis and maturation, resulting in changes that affect both structure and function of multiple organ systems, e.g., the respiratory and neurological systems (13). When normal development is altered, the early effects may persist into adult life, magnifying the public health impact.
Although the range of health outcomes that may ultimately be of interest may have disparate vulnerability factors and natural histories (e.g., many do not manifest until later childhood or even into adulthood), most will have their roots very early in development, including prenatally—a notion grounded in the “developmental origins of chronic disease hypothesis (12, 14, 15).” Studies suggest that characteristics of the in utero environment, independent of genetic susceptibility, influence embryonic and fetal development, including neurological, endocrine, cardiovascular, respiratory, and immune development. Early misconceptions that organ systems finished their overall development at the end of the second trimester gave rise to the inaccurate concept that exposure to factors such as alcohol after this time would not result in developmental toxicity. We now know that these patterns are much more complex and the windows of vulnerability much larger. Specific biological events occur in each trimester of pregnancy, changing susceptibility to environmental factors across time, agent, and dose. Since scientists demonstrated that environmental impacts during the second trimester could result in specific birth defects (16), they recognized that when the patterns of proliferation and differentiation were tracked, a prediction of specific windows of susceptibility was evident.
Many other concepts of developmental toxicology are known for this period. The developing fetus and young child are uniquely vulnerable because their respiratory, immune, neuroendocrine, and xenobiotic detoxification systems are in the process of development throughout pregnancy and early childhood (17, 18). For example, we know the conceptus in utero, and young children differentially gain the ability to metabolize environmental chemicals; therefore, knowing which metabolizing enzymes are present at various times in utero and post-natally is significant in understanding when and how chemicals impact development. Such knowledge can identify the form and potential for chemicals to impact development. For example, this information is used clinically to treat the neonate when drug metabolism activity levels exceed adult human levels of metabolism (requiring larger-than-adult drug doses for some clinical treatments). This means that differential times of exposure to the same toxicant are in reality, very different. For the NCS, we need to ensure that sufficient assessments and biospecimens are available to support these observations and interpret intrauterine exposures.
Adolescence represents another key transitional period in the life course that is vulnerable to environmental programming (19–22). The adolescent period corresponds to the attainment of adult height and sexual maturity. Key homeostatic systems are going through changes during the process of sexual maturation, involving complex interactions between the central nervous system (CNS), hormone-secreting organs, and immune function, all of which can be affected by environmental factors (23, 24). The period from adolescence through early adulthood may also be a time of susceptibility to environmental toxins/toxicants because organ systems (e.g., the lung, brain) are in the final phase of growth and maturation, and because adolescence is a period of rapid social and emotional change.
The NCS process was also guided by a life course approach, which considers both the more immediate and longer-term effects of physical and social exposures during all periods of development (e.g., gestation, childhood, adolescence, early adulthood, and later adult life) (3). Recommended age-appropriate measures and timing of assessments were guided by our understanding of differential vulnerabilities to disease between individuals depending on the developmental stage when exposure occurs (e.g., childhood, adolescence, young adulthood, and later adult life). Moreover, adverse exposures during critical periods may have concurrent effects, latent effects (not observed for a number of years) and cumulative effects (adding up as subjects age) that negatively impact health outcomes and trajectories over the life course (25). Chronic exposures recurring or persisting over multiple developmental periods should also be considered (cumulative chemical exposures, ongoing traumas, persistent poverty, etc.).
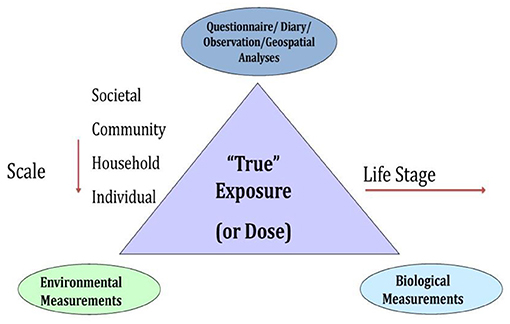
A key challenge was, thus, to identify where, when, and what measures, environmental samples, and biospecimens should be collected to characterize potential exposure factors and effects of exposures across the life course in a reasonably comprehensive manner. External exposure assessment relies on measuring factors broadly characterized, such as chemical and non-chemical stressors, diet, and behaviors (Figure 2). Moreover, the built environment influences each of these exposure classifications. Combined approaches, some overlapping, including survey measures, observational measures, direct monitoring approaches with laboratory analysis, and geospatial analysis leveraging extant data gathered for other purposes, can be used to characterize the environment (see Figure 3, and summarized below). It is important to identify at what level of biological organization and life stage to collect such assessments. Figure 3 illustrates the need to look at this at various ecological levels, for example, at the individual, family, household, and community level in order to analyze and interpret environmental profiles.
Overview
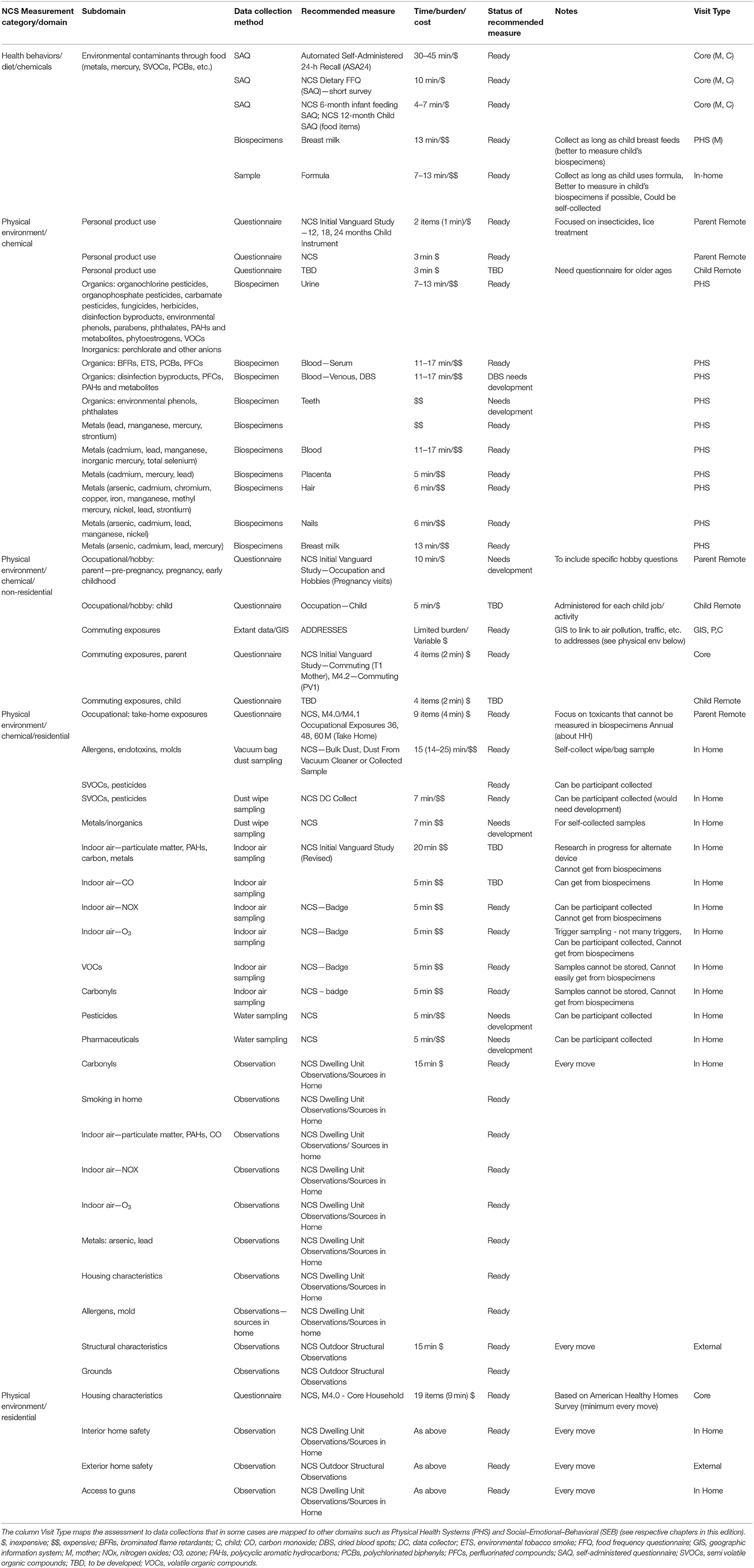
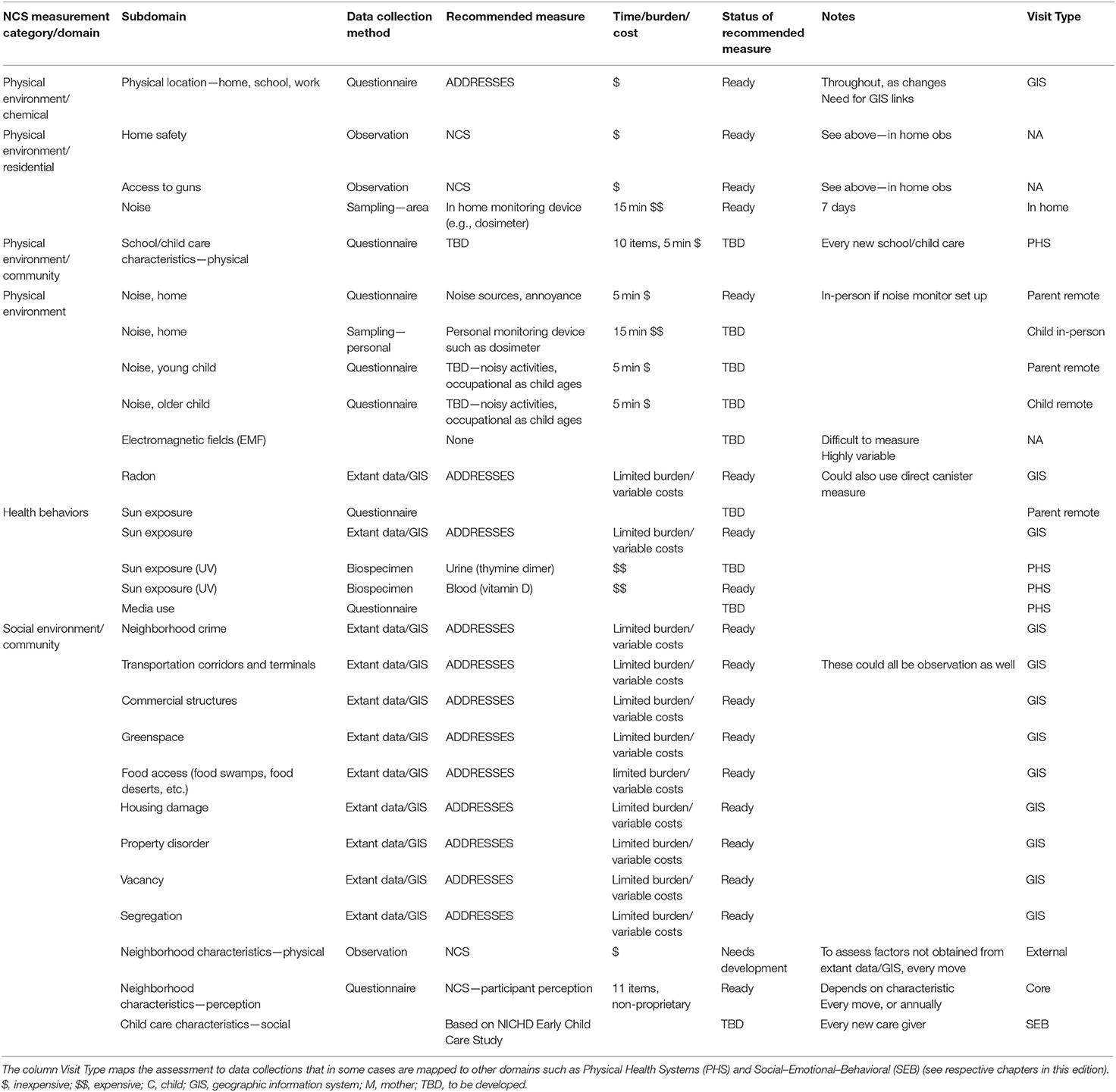
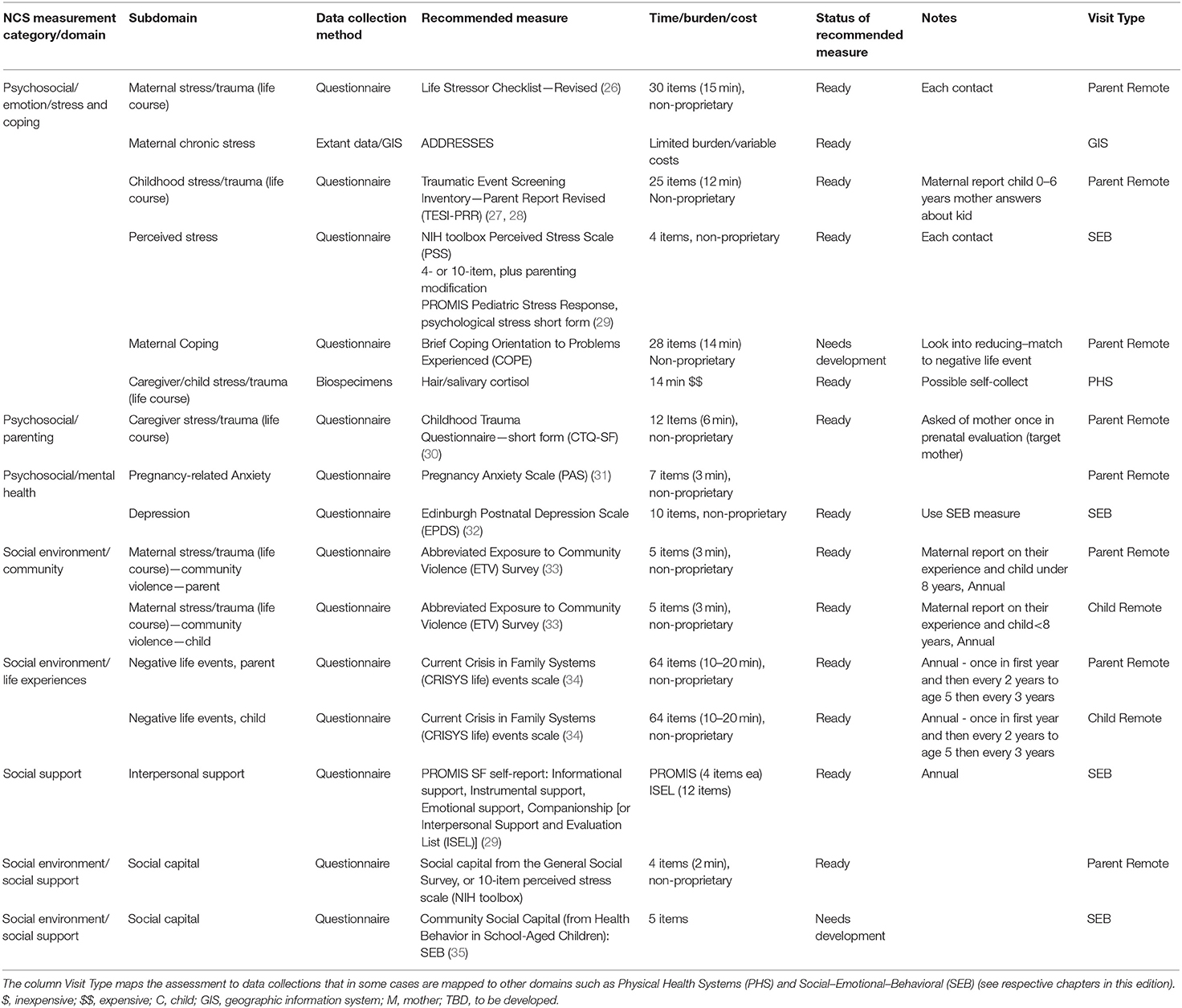
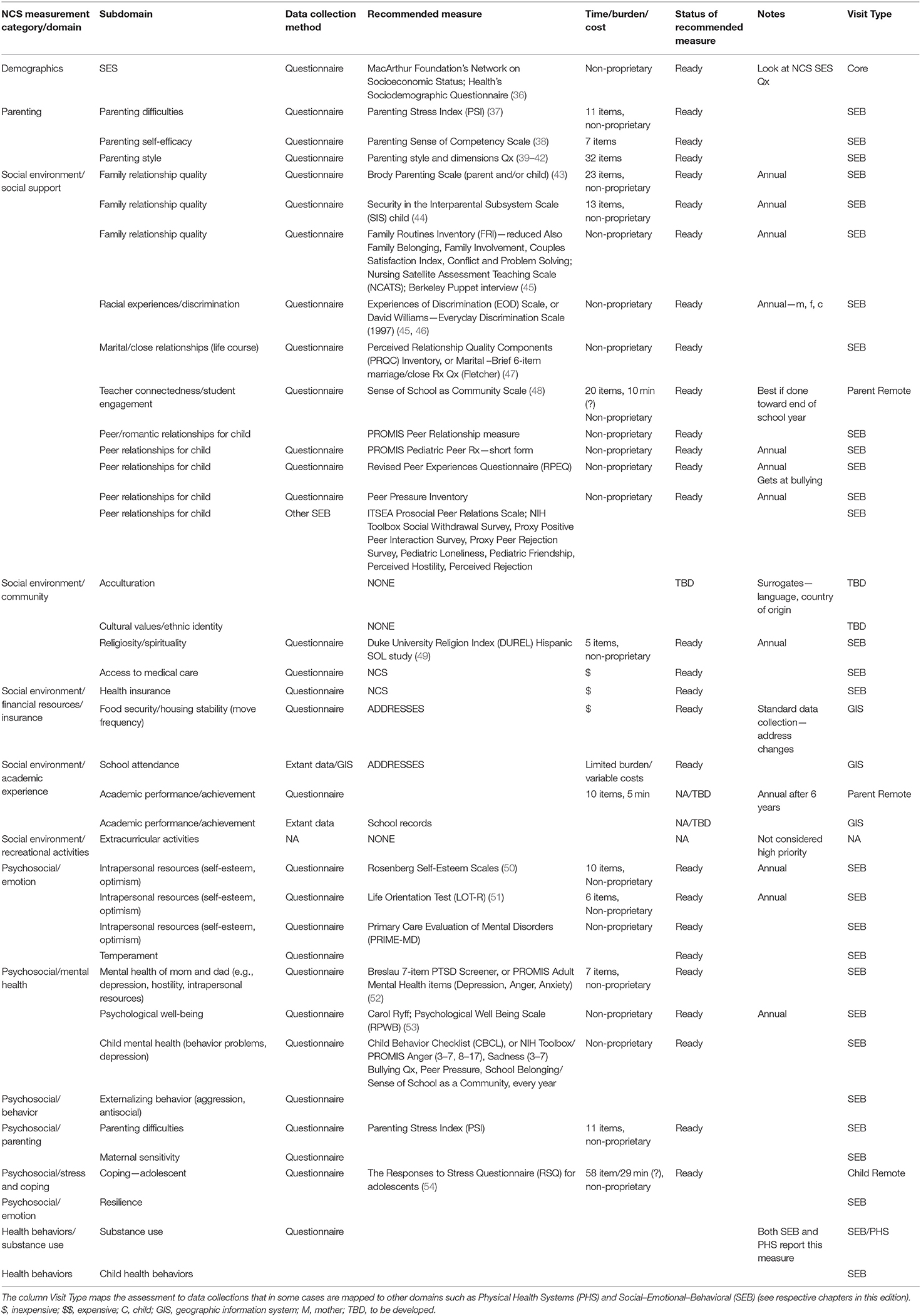
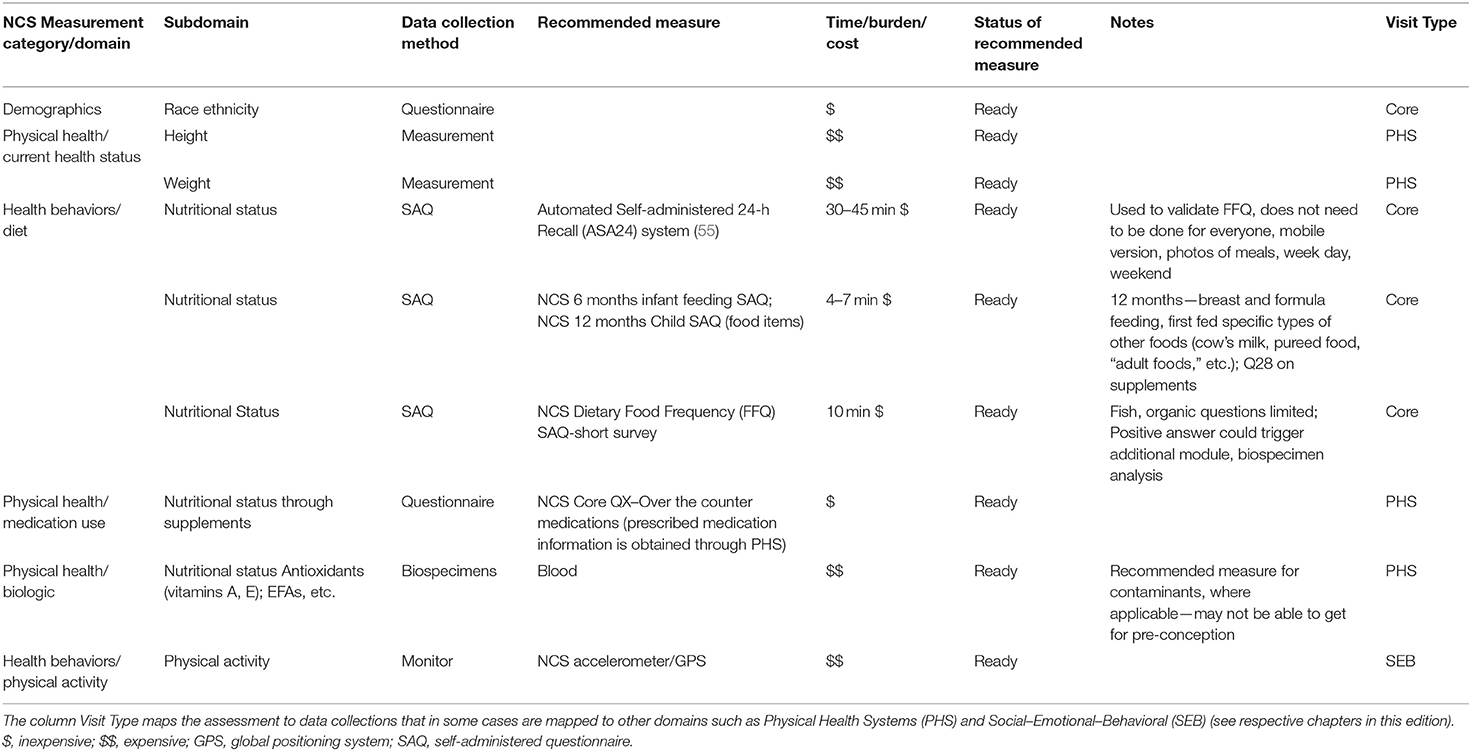
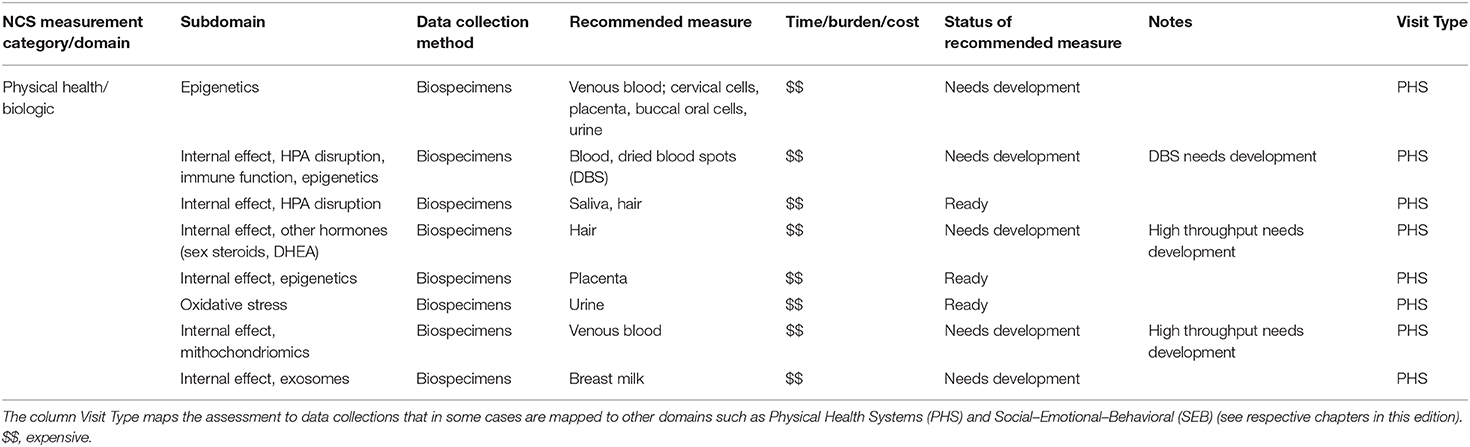
The National Children's Study (NCS), Health Measurement Network (HMN), and Environmental Domain Working Group addressed the need for an environmental measurement framework to answer critical questions regarding children's health by developing recommendations for assessment of environmental exposures across the life stage within the NCS. The attached grid (Tables 1–6) summarizes the group's recommendations of measurement for the following five domains:
1. Chemical and biological exposures including organic chemicals (pesticides, organochlorine pesticides, organophosphate pesticides and metabolites, carbamate pesticides, fungicides, herbicides, brominated flame retardants, disinfection byproducts, environmental tobacco smoke markers, environmental phenols, parabens, perfluorinated compounds, bisphenol A and phthalates, polychlorinated biphenyls, polycyclic aromatic hydrocarbon and metabolites, and volatile organic hydrocarbons), inorganic chemicals (metals, and perchlorate and other anions), and biological exposures (allergens and mold, and microbes);
2. Physical environment exposures including community design, physical safety, access to food resources, radiation exposure, ultraviolet radiation, radon, and noise exposure;
3. Stress including social support and coping;
4. Social determinants of health including socioeconomic status, family relationship quality, parent/caregiver mental health, parenting difficulties, attachment, child care/school characteristics, community violence, social capital, school environment, peers and neighborhood, and mentoring; and
5. Modifying factors including diet, obesity, and physical activity. Sources of information collected in the Grid are summarized below.
This narrative discusses the following:
1) What do these measurements represent? This narrative encompasses three different views: discussion of the state of the art for exposure measurements, the importance of an emphasis on biomarkers for exposure assessment as these are the most direct estimate exposure and our view toward obtaining maximum amount of information from specific biospecimens and exposure techniques. For example, a biospecimen that can be collected once and then analyzed for multiple exposures hold more value than a sample, which can only be used to assess one exposure. It has been challenging for most cohorts to determine what measures, when and where, and how. This is especially true across life stage where specimens may be limited, and participant burden is an important consideration.
2) How can the narrative and grid be used? The narrative and the grid are divided into domains (listed above), measurement categories, and subdomains. Across all of these, the group assessed data collection method, recommended measures, time, burden and cost, status of recommended measure, and life course assessment timing. Data collection methodologies include questionnaires, biospecimen collection, environmental samples, observations, and extant data usage. Each data collection methodology is linked with a recommended measure, including a specific biospecimen or questionnaire. In order to allow the grid to be relevant to future studies, the status of the recommended measure was also assessed. This allowed for inclusion of methodologies that are not ready for implication yet, but show significant promise for future work. All of these measures are discussed in the context of developmental timing for optimal assessment. The grid was designed for comprehensive longitudinal cohort studies over 21 years. To use this moving forward, there is a context and purpose that could be applied to pull out subsets of the assessments to answer specific questions and or hypothesis. Also, as one reviews cohort studies, such measures and their consistency could be used to facilitate the cross study interpretation and translation.
3) What types of measurements and tools are employed in the narrative and grid?
Survey measures. Survey measures such as those listed below are the least expensive construct type, although they do not always provide quantitative exposures and can contribute significantly to participant time burden. They may be administered by study staff or self-administered by the respondent. They may be administered by direct questioning, or privately via computer. Technological advances also allow these to be completed via web or smartphone.
Questionnaires. Questionnaires ask the respondent questions about a given topic. The questions may address themselves, their family or friends, their child, or their home, school, or neighborhood/community. Examples of exposure topics for which questionnaires are recommended include stress, family relationship quality, use of pesticides and consumer products, occupational exposures, sources and perception of noise, and home, school, and neighborhood/community characteristics.
Diaries. Diaries ask the respondent to answer the same questions over time. These are usually left with the respondent to complete at specified time points. Examples of exposure topics for which diaries are recommended include diet and time-place activities.
Observational measures. Observations can be made by a data collector or the participant. In either case, these survey measures cost more than questionnaires or diaries. Observations done by a data collector add staff costs, while observations made by a participant may require educating the participant and may be biased. The types of environmental exposure information that can be captured by observations include indoor and outdoor environmental and safety features of the home, child care/school, or workplace. It may also be difficult to obtain permission to conduct observations of child care/school and work settings.
Sample collection with laboratory analysis. Environmental samples are collected to determine levels of chemical or environmental toxicants in various media (e.g., dust, water, air, soil, food). Sample collection is relatively inexpensive where samples can be left in storage for future case-control study analyses. However, some sample types cannot be stored and must be analyzed immediately. While lab analysis can be costly, some analytical methods can measure many chemicals (see Multiple Chemical Assessments discussion below). There is also the issue that just because a chemical is in the environment does not mean it gets into a child's body and impacts health. Sample collection is generally only recommended where there is no other way to assess the exposure, e.g., via survey methods, extant data, or biospecimen collection. As such, the types of exposures for which sample collection is recommended include indoor air particulate matter and air oxidants, and semi-volatile chemicals via vacuum bag dust and dust wipe sample collection.
Direct monitoring. Direct monitoring approaches typically involve devices, which measure an exposure directly and integrate the exposure over time. Examples include measurement of air oxidants via sensors, measurement of noise levels, and physical activity accelerometers. These types of devices can be moderately to very expensive upfront, but do not require subsequent laboratory analysis, and can be left in a home, other environment, or with a participant to determine exposures over specified time periods. Sensor and computer application technologies are evolving rapidly, and smartphone compatible sensors may soon be available.
Biomarkers. Where available, biomarker measures are generally considered more direct exposure measures than survey, ambient monitoring, and sampling approaches. Options for biomarker measures are discussed in more detail below.
Geospatial analysis. Geospatial analyses link addresses to extant data, e.g., EPA air monitoring data or local crime statistics, to estimate exposures to individuals in various locations, e.g., the home/neighborhood, school, or work. Geospatial modeling has been utilized to characterize a number of environmental factors including local food environments (56–58), built environment for physical activity (59), ambient pollution (60), water contamination, and spatio-temporal patterns in crime (61). There has also been a call for the need to include disability-specific items in measures of the built environment (62). While this type of data requires no input from or burden to the participant, costs for developing and maintaining these databases and analyses can be substantial.
Exposure models. Chemical exposures can be modeled from available information, including extant data, findings from survey measures and observations, sampling data from a subset of other participants, etc.
In the subsequent sections of this report, we describe the domains used in the grid in detail. Chemical and Biological Exposures, Physical Environment, Social Stress, Social Determinants of Health and Effect Modifiers are all discussed below with special care to address subdomains and recommended measures included in the grid.
Grid Introduction
The Health Measurement Network (HMN) Environmental Domain Working Group developed recommendations for assessment of environmental exposures in the National Children's Study (NCS). The grid summarizes the group's recommendations of measurement for the following five categories: (1) Chemical and biological exposures including organic chemicals (pesticides, organochlorine pesticides, organophosphate pesticides, and metabolites, carbamate pesticides, fungicides, herbicides, brominated flame retardants, disinfection byproducts, environmental tobacco smoke markers, environmental phenols, parabens, perfluorinated compounds, BPA and phthalates, polychlorinated biphenyls, polycyclic aromatic hydrocarbon and metabolites, and volatile organic hydrocarbons), inorganic chemicals (metals, and perchlorate and other anions), and biological exposures (allergens and mold, and microbes); (2) physical environment exposures including community design, physical safety, access to food resources, radiation exposure, ultraviolet radiation, radon, and noise exposure; (3) stress including social support and coping; (4) social determinants of health including socioeconomic status, family relationship quality, parent/caregiver mental health, parenting difficulties, attachment, child care/school characteristics, community violence, social capital, school environment, peers and neighborhood, and mentoring; and (5) modifying factors including diet, obesity, and physical activity. The recommended measures are given from pre-conception through young adulthood. For each subdomain within a category, the grid specifies the recommended data collection method, recommended measure, time/burden/cost, prioritization of source of information, status of the recommended measure, notes about the recommendation as well as the visit type and time of the measurement.
Importance of the Grid and How It Could Be Used
Based on the discussions above, we developed a grid for assessment of both chemical and non-chemical exposomes (see Tables 1–6).
1. Examples of specific measures, how and when they would be used in a life course cohort study.
2. Identifies state of the art methods and identifies where further development is needed.
3. Consolidates measures for chemical, physical, and social determinants of health in one framework across “environment.”
4. Inclusion of time, burden, and cost also provides insight for alternate measures.
5. Provides a common platform for integrating exposure measures across multiple cohorts and studies.
The grid can also be used to prioritize measures of exposure. Factors to consider in selecting environmental measurements for a specific cohort study include the research question to be answered, budget, population, and exposure scope. Prioritization criteria include value of information obtained from a particular environmental measure, direct relationship of measurement to receptor (primary vs. secondary markers), and ability to provide co-exposure solutions, i.e., the same measurement could provide information on multiple chemicals and exposure routes. For example, integrated measures that show exposure of a chemical over time might have higher prioritization because of the ability to assess the temporality of exposure, especially during pregnancy when more direct measures of exposure profiles may be less feasible. There are many measures that are “ready,” i.e., the methodology is developed, and the recommended measure is available for immediate use. Other prioritization factors include the validation, stability, specificity, and sensitivity of the assays to be used. Although not a key focus of this assessment, the limit of detection is a key element that needs to be matched with the research question. Ease of collection and participant burden is a factor in all prioritization schemes and can vary dramatically between study constructions. This grid and these prioritization criteria can be used to help identify the optimal assessment methods for children's environmental health cohorts.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SV: manuscript facilitator and editor, wrote some sections, and managed tables. MD: NIH investigator leading effort and reviewed all. EC: wrote section on behavioral instruments. TM: wrote sections on tooth analysis. EF: technical support and reviewer. SB: wrote section on built environment. MS: researched references for psychosocial assessment methods. RW: team lead, wrote introductory sections and figures. All authors: contributed to the article and approved the submitted version.
Funding
This research was conducted under the NICHD National Children's Study. Each author worked under a different contract.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to express thanks to others who supported this work, including Elizabeth Boyle, Beryl Carew, and Ashley Martin. In addition, we wish to thank the staff at the National Children's Study Centers who piloted the Vanguard procedures, providing valuable feedback. We also thank the many women and their families who volunteered to participate in the Vanguard pilot study.
References
1. Miller GW, Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci. (2013) 137:1–2. doi: 10.1093/toxsci/kft251
3. Ben-Shlomo Y. Rising to the challenges and opportunities of life course epidemiology. Int J Epidemiol. (2007) 36:481–3. doi: 10.1093/ije/dym116
4. Wright RJ. Moving towards making social toxins mainstream in children's environmental health. Curr Opin Pediatr. (2009) 21:222–9. doi: 10.1097/MOP.0b013e3283292629
5. Krech R. Healthy public policies: looking ahead. Health Promot Int. (2011) 26 (Suppl. 2):ii268–72. doi: 10.1093/heapro/dar066
6. Kuh D. Life course epidemiology. J Epidemiol Commun Health. (2003) 57:778–83. doi: 10.1136/jech.57.10.778
7. Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments—the long-term consequences for disease risk. Early Hum Dev. (2005) 81:51–9. doi: 10.1016/j.earlhumdev.2004.10.003
8. Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab. (2008) 22:1–16. doi: 10.1016/j.beem.2007.07.009
9. Rzeczkowska PA, Hou H, Wilson MD, Palmert MR. Epigenetics: a new player in the regulation of mammalian puberty. Neuroendocrinology. (2014) 99:139–55. doi: 10.1159/000362559
10. Shaughnessy DT, McAllister K, Worth L, Haugen AC, Meyer JN, Domann FE, et al. Mitochondria, energetics, epigenetics, and cellular responses to stress. Environ Health Perspect. (2014) 122:1271–8. doi: 10.1289/ehp.1408418
11. Senaldi L, Smith-Raska M. Evidence for germline non-genetic inheritance of human phenotypes and diseases. Clin Epigenet. (2020) 12:136. doi: 10.1186/s13148-020-00929-y
12. Kajantie E. Fetal origins of stress-related adult disease. Ann NY Acad Sci. (2006) 1083:11–27. doi: 10.1196/annals.1367.026
13. Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. (2007) 447:433–40. doi: 10.1038/nature05919
14. Barker DJP. A new model for the origins of chronic disease. Med Health Care Philos. (2001) 4:31–5. doi: 10.1023/A:1009934412988
15. Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. (2001) 30:15–23. doi: 10.1093/ije/30.1.15
16. Wilson JG. Environment and Birth Defects [by] James G. Wilson. Environmental Sciences Research Report. New York, NY: Academic Press (1973).
17. Scheuplein R, Charnley G, Dourson M. Differential sensitivity of children and adults to chemical toxicity. Regulat Toxicol Pharmacol. (2002) 35:429–47. doi: 10.1006/rtph.2002.1558
18. Dourson M, Charnley G, Scheuplein R. Differential sensitivity of children and adults to chemical toxicity. Regulat Toxicol Pharmacol. (2002) 35:448–67. doi: 10.1006/rtph.2002.1559
19. Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Trans Psychiatry. (2013) 3:e238. doi: 10.1038/tp.2013.7
20. Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Front Synaptic Neurosci. (2012) 4:3. doi: 10.3389/fnsyn.2012.00003
21. Efrat M, Tepper S, Birk RZ. From fat cell biology to public health preventive strategies – pinpointing the critical period for obesity prevention. J Pediatr Endocrinol Metab. (2013) 26:197–209. doi: 10.1515/jpem-2012-0379
22. Alberga AS, Sigal RJ, Goldfield G, Prud'Homme D, Kenny GP. Overweight and obese teenagers: why is adolescence a critical period? Pediatr Obes. (2012) 7:261–73. doi: 10.1111/j.2047-6310.2011.00046.x
23. Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev. (2011) 35:1704–12. doi: 10.1016/j.neubiorev.2011.04.003
24. Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophrenia Bull. (2011) 37:514–23. doi: 10.1093/schbul/sbr034
25. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. (2002) 31:285–93. doi: 10.1093/ije/31.2.285
26. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, editors. Assessing Psychological Trauma and PTSD. New York, NY: Guilford Press (1997). p. 192–238.
27. Ippen CG, Ford J, Acker M, Bosquet M, et al. Traumatic events screening inventory - parent report revised. Ribbe D. (1996) Psychometric review of Traumatic Event Screening Instrument for Children (TESI-C). In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. (2002). p. 386–7.
28. Ribbe D. Psychometric review of traumatic event screening instrument for children (TESI-C). In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Derwood, MD: Sidran Press (1996). p. 386–7.
29. PROMIS (2018). Available online at: http://www.healthmeasures.net/explore-measurement-systems/promis (accessed March 28, 2018).
30. Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Neglect. (2003) 27:169–90. doi: 10.1016/S0145-2134(02)00541-0
31. Brunton RJ, Dryer R, Saliba A, Kohlhoff J. Pregnancy anxiety: a systematic review of current scales. J Affect Disord. (2015) 176:24–34. doi: 10.1016/j.jad.2015.01.039
32. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. N Engl J Med. (1987) 347:194–9. doi: 10.1192/bjp.150.6.782
33. Selner-O'Hagan MB, Kindlon DJ, Buka SL, Raudenbush SW, Earls FJ. Assessing exposure to violence in urban youth. Assoc Child Psychol Psychiatry. (1998) 39:215–24. doi: 10.1017/S002196309700187X
34. Berry C, Quinn K, Shalowitz M, Wolf R. Validation of the crisis in family systems-revised, a contemporary measure of life stressors. Psychol Rep. (2001) 88 (3 Pt 1):713–24. doi: 10.2466/pr0.2001.88.3.713
35. Mytton OT, Townsend N, Rutter H, Foster C. Green space and physical activity: an observational study using health survey for England data. Health Place. (2012) 18:1034–41. doi: 10.1016/j.healthplace.2012.06.003
36. Lawrence E, Pederson A, Bunde M, Barry RA, Brock RL, Fazio E, et al. Objective ratings of relationship skills across multiple domains as predictors of marital satisfaction trajectories. J Soc Person Relatsh. (2008) 25:445–66.
38. Wandersman G-W. Parenting Sense of Competency Scale (1978). Available online at: https://socialsuitehq.com/product/parenting-sense-of-competence-scale-psoc/
39. Olivari MG, Tagliabue S, Confalonieri E. Parenting style and dimensions questionnaire: a review of reliability and validity. Marriage Family Rev. (2013) 49:465–90. doi: 10.1080/01494929.2013.770812
40. Parisette-Sparks A, Bufferd SJ, Klein DN. Parental predictors of children's shame and guilt at age 6 in a multimethod, longitudinal study. J Clin Child Adolesc Psychol. (2017) 46:721–31. doi: 10.1080/15374416.2015.1063430
41. Miragoli S, Camisasca E, Di Blasio P, Milani L, Ionio C, Gizzi N, et al. Child abuse potential inventory in Italy: a comparative study of abusive and nonabusive parents. J Child Custody. (2016) 13:289–306. doi: 10.1080/15379418.2016.1250145
42. Tagliabue S, Olivari MG, Miranda MC, Affuso G, Bacchini D, Confalonieri E. Memories of parenting styles and communicative processes in adolescence. Family Sci. (2015) 6:389–93. doi: 10.1080/19424620.2015.1110192
43. Brody GH, Flor DL. Maternal resources, parenting practices, and child competence in rural, single-parent African American families. Child Dev. (1998) 69:803–16. doi: 10.1111/j.1467-8624.1998.tb06244.x
44. Davies PT, Forman EM, Rasi JA, Stevens KI. Assessing children's emotional security in the interparental relationship: the security in the interparental subsystem scales. Child Dev. (2002) 73:544–62. doi: 10.1111/1467-8624.t01-1-00512
45. Jensen EW, James SA, Boyce WT, Hartnett SA. The family routines inventory: development and validation. Soc Sci Med. (1983) 17:201–11. doi: 10.1016/0277-9536(83)90117-X
46. Williams DR. Measuring Discrimination Resource (2016). Available online at: https://scholar.harvard.edu/files/davidrwilliams/files/measuring_discrimination_resource_june_2016.pdf
47. Fletcher GJ, Simpson JA, Thomas G. The measurement of perceived relationship quality components: a confirmatory factor analytic approach. Pers Soc Psychol Bull. (2000) 26:340–54. doi: 10.1177/0146167200265007
48. Wilfried A, Ditte L. The sense of community in school scale (SCSS). J Workplace Learn. (2012) 24:245–55. doi: 10.1108/13665621211223360
49. Koenig HG, Büssing A. The Duke University Religion Index (DUREL): a five-item measure for use in epidemological studies. Religions. (2010) 1:78–85. doi: 10.3390/rel1010078
50. Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princceton University Press (1965).
51. Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a re-evaluation of the life orientation test. J Pers Soc Psychol. (1994) 67:1063–78. doi: 10.1037/0022-3514.67.6.1063
52. Kimerling R, Ouimette P, Prins A, Nisco P, Lawler C, Cronkite R, et al. Brief report: utility of a short screening scale for DSM-IV PTSD in primary care. J Gen Intern Med. (2006) 21:65–7. doi: 10.1111/j.1525-1497.2005.00292.x
53. Henn CM, Hill C, Jorgensen LI. An investigation into the factor structure of the Ryff Scales of Psychological Well-Being. SA J Industr Psychol. (2016) 2016:42. doi: 10.4102/sajip.v42i1.1275
54. Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H. Responses to stress in adolescence: measurement of coping and involuntary stress responses. J Consult Clin Psychol. (2000) 68:976–92. doi: 10.1037/0022-006X.68.6.976
55. Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, et al. The automated self-administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. (2012) 112:1134–7. doi: 10.1016/j.jand.2012.04.016
56. Charreire H, Casey R, Salze P, Simon C, Chaix B, Banos A, et al. Measuring the food environment using geographical information systems: a methodological review. Public Health Nutr. (2010) 13:1773–85. doi: 10.1017/S1368980010000753
57. Ohri-Vachaspati P, Leviton LC. Measuring food environments: a guide to available instruments. Am J Health Promot. (2010) 24:410–26. doi: 10.4278/ajhp.080909-LIT-190
58. Kelly B, Flood VM, Yeatman H. Measuring local food environments: an overview of available methods and measures. Health Place. (2011) 17:1284–93. doi: 10.1016/j.healthplace.2011.08.014
59. Brownson RC, Hoehner CM, Day K, Forsyth A, Sallis JF. Measuring the built environment for physical activity. Am J Prevent Med. (2009) 36:S99–123.e12. doi: 10.1016/j.amepre.2009.01.005
60. Gryparis A, Coull BA, Schwartz J, Suh HH. Semiparametric latent variable regression models for spatiotemporal modelling of mobile source particles in the greater Boston area. J R Statist Soc C. (2007) 56:183–209. doi: 10.1111/j.1467-9876.2007.00573.x
61. Davies TP, Bishop SR. Modelling patterns of burglary on street networks. Crime Sci. (2013) 2:10. doi: 10.1186/2193-7680-2-10
Keywords: exposure assessment, longitudinal research, environmental exposures, pediatric longitudinal research, psychosocial environment
Citation: Viet SM, Dellarco M, Chen E, McDade T, Faustman E, Brachvogel S, Smith M and Wright R (2021) Recommendations for Assessment of Environmental Exposures in Longitudinal Life Course Studies Such as the National Children's Study. Front. Pediatr. 9:629487. doi: 10.3389/fped.2021.629487
Received: 15 November 2020; Accepted: 25 February 2021;
Published: 29 April 2021.
Edited by:
Jerry Slotkin, University of Delaware, United StatesReviewed by:
Lilach Soreq, University College London, United KingdomJean Golding, University of Bristol, United Kingdom
Copyright © 2021 Viet, Dellarco, Chen, McDade, Faustman, Brachvogel, Smith and Wright. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susan Marie Viet, susanviet@westat.com
 Susan Marie Viet
Susan Marie Viet Michael Dellarco2
Michael Dellarco2  Thomas McDade
Thomas McDade Elaine Faustman
Elaine Faustman Rosalind Wright
Rosalind Wright