Emotional memory formation under lower versus higher stress conditions
- 1 Department of Psychology and the Brain and Behavior Research Center, University of Haifa, Haifa, Israel
- 2 Department of Neurobiology and Ethology, University of Haifa, Haifa, Israel
- 3 Institute for the Study of Affective Neuroscience, University of Haifa, Haifa, Israel
An exposure to stress can enhance memory for emotionally arousing experiences. The phenomenon is suggested to be amygdala-dependent and in accordance with that view the amygdala was found to modulate mnemonic processes in other brain regions. Previously, we illustrated increased amygdala activation and reduced activation of CA1 following spatial learning under higher versus lower stress conditions. When spatial learning was followed by reversal training interference, impaired retention was detected only under higher stress condition. Here we further evaluate the potential implications of the difference in the level of amygdala activation on the quality of the memory formed under these stress conditions. We attempted to affect spatial memory consolidation under lower or higher stress conditions by either introducing a foot shock interference following massed training in the water maze; by manipulating the threshold for acquisition employing either brief (3 trials) or full (12 trials) training sessions; or by employing a spaced training (over 3 days) rather than massed training protocol. The current findings reveal that under heightened emotionality, the process of consolidation seems to become less effective and more vulnerable to interference; however, when memory consolidation is not interrupted, retention is improved. These differential effects might underlie the complex interactions of stress, and, particularly, of traumatic stress with memory formation processes.
Introduction
It has been suggested that emotional arousal may enhance one or more of several memory stages, including memory acquisition, consolidation, and retrieval (Cahill and McGaugh, 1995; Cahill et al., 1996; Buchanan and Lovallo, 2001; Dolcos et al., 2004, 2005). It is argued that the memory-enhancing effects of emotional arousal reflect the influence of the amygdala, and specifically, the basolateral amygdala (BLA), on mnemonic processes occurring in medial temporal lobe (MTL) structures, including the hippocampus and associated parahippocampal regions (i.e., entorhinal, perirhinal, and parahippocampal cortices; McGaugh et al., 1996; McGaugh, 2004). These regions are implicated in the generation of declarative memory in humans and of spatial memory in animals (Morris et al., 1982; Squire and Zola-Morgan, 1991).
Spatial learning in the Morris water maze is an aversively motivated experience and acquiring this task is a stressful event for the animal, as indicated by increased corticosterone release during training (Sandi et al., 1997; Schaaf et al., 1999; Akirav et al., 2001; Akirav et al., 2004).
The level of emotionality of this learning experience could be further manipulated by altering the water temperature in the maze. Previously, it was demonstrated that spatial learning under warm water (WW) versus cold water (CW) in the Morris water maze, resulted in a different acquisition pattern (Sandi et al., 1997) and in higher corticosterone levels in the CW group (Sandi et al., 1997; Akirav et al., 2001, 2004), implying that it is a higher stress condition.
Using ERK2 as a biochemical marker, the BLA was found to be activated only when learning the water maze task under cold water with higher levels of stress (Akirav et al., 2001). Recently, we showed a differential activation pattern of CREB in the hippocampus (dorsal CA1), BLA and entorhinal cortex (EC) following learning a spatial task under warm water (24 ± 1 °C) condition versus cold water (19 ± 1°C) condition. Increased activation of the hippocampus was detected following spatial learning under WW compared to CW condition, whereas enhanced BLA activation was found following spatial learning under CW condition compared to the WW condition (Kogan and Richter-Levin, 2008).
As extensive evidence from human (Adolphs et al., 1997; Hamann et al., 1999; Quevedo et al., 2003; Dolcos et al., 2004, 2005; Smith et al., 2006) and animal (McGaugh et al., 1996; McGaugh, 2004) studies suggests that enhanced amygdala activation is correlated with and may contribute to enhanced long-term memory for emotional events, it can be expected that increased activation of the BLA following the training in the Morris water maze under CW condition with higher levels of corticosterone released (Sandi et al., 1997; Akirav et al., 2001, 2004), would lead to enhanced long-term memory for the platform location compared to long-term memory generated under WW conditions.
Contrary to this expectation, we have found that reversal training interference interrupted the memory of CW animals (i.e., with increased BLA activation, Kogan and Richter-Levin, 2008). It has thus been suggested that BLA activation could be beneficial to some, but not all, parameters of memory formation. For example, BLA activation could enhance the long-term memory trace for the event, but at the price of rendering the consolidation process more vulnerable to interference.
We set out to examine the effects of training under warm and cold water conditions on several independent parameters of memory quality. We tested resistance to interference introduced shortly after the training during the initial stages of consolidation. We further examined the effects of altering levels of emotionality during acquisition on the threshold for acquiring efficient spatial memory, with brief, 3-trials, and full, 12-trials, sessions of training. Finally, the formation of an effective long-term spatial memory was examined by employing spaced training protocol, which is considered a more efficient training protocol than the massed training protocol. We predicted that subjection to the CW condition would lead to a stronger memory trace (as indicated by spatial memory retention) that would be more resistant to manipulations affecting memory consolidation.
Materials and Methods
Subjects
Male Wistar Hanover rats (Harlan, Jerusalem) weighing 250–350 g. were maintained five per cage on a 12 h light/dark cycle with water and a laboratory rodent chow ad libitum. The Haifa University Animal Care and Use Committee approved the study and adhered to the NIH Guide for the Care and Use of Laboratory Animals.
Behavioral Procedures
Morris water maze task
The water maze (Morris et al., 1982) consisted of a featureless circular pool (diameter 1.7 m, rim 50 cm high) that was painted black and filled with water to a depth of 30 cm. A hidden black escape platform (12 cm × 12 cm) was placed in one of its quadrants such that its top lay 2 cm beneath the water surface. The only spatial landmarks for the animals to locate an escape platform were located outside the maze. Each trial was initiated by placing the animal in one of the three other quadrants near the wall of the pool. Animals were allowed to search for the escape platform for a maximum of 60 s. Those that failed to locate the platform within this period were guided to it by the experimenter. Animals remained on the platform for 15 s prior to removal from the maze. The escape latency was measured with a stopwatch.
Water temperature was used as a means of controlling emotionality level of the learning environment as described before (Akirav et al., 2001; Kogan and Richter-Levin, 2008) with WW (24 ± 1°C) providing a lower stress condition, and CW (19 ± 1°C) providing a higher stress condition.
Electrical foot shock
Apparatus consisted of a small cube-like chamber (31 cm × 31 cm × 31 cm) with a metal grid floor connected to a computer-controlled electrical shocker device (Solid State Shocker/Scrambler, Model no. 113–33, Lehigh Valley Electronics Ltd, Lehigh Valley, PA, USA).
The animals were exposed to a 5 min session of 10 unconditioned electric foot shocks (1 s, 0.5 mA) with inter-trial interval of 29 s.
Experiment 1 – the effects of interference during consolidation on spatial memory formation
On the first day, the animals were subjected to spatial learning in the Morris water maze utilizing a massed training protocol that consisted of 12 trials under the WW or the CW conditions.
These groups were each divided into the two subgroups: groups without interference (WW, n = 10 and CW, n = 8), and groups with interference (WW_I; n = 9 and CW_I; n = 12). Electrical foot shocks (10 shocks of 1 s, 0.5 mA) that served as interference to spatial memory consolidation process were induced 1 h following the end of training in the Morris water maze. On the third day, retention was tested by a relearning session; the animals were introduced to two trials in the water maze with platform located in the original quadrant beneath the water surface. Inter-trial interval was 1 min (Figure 1A).
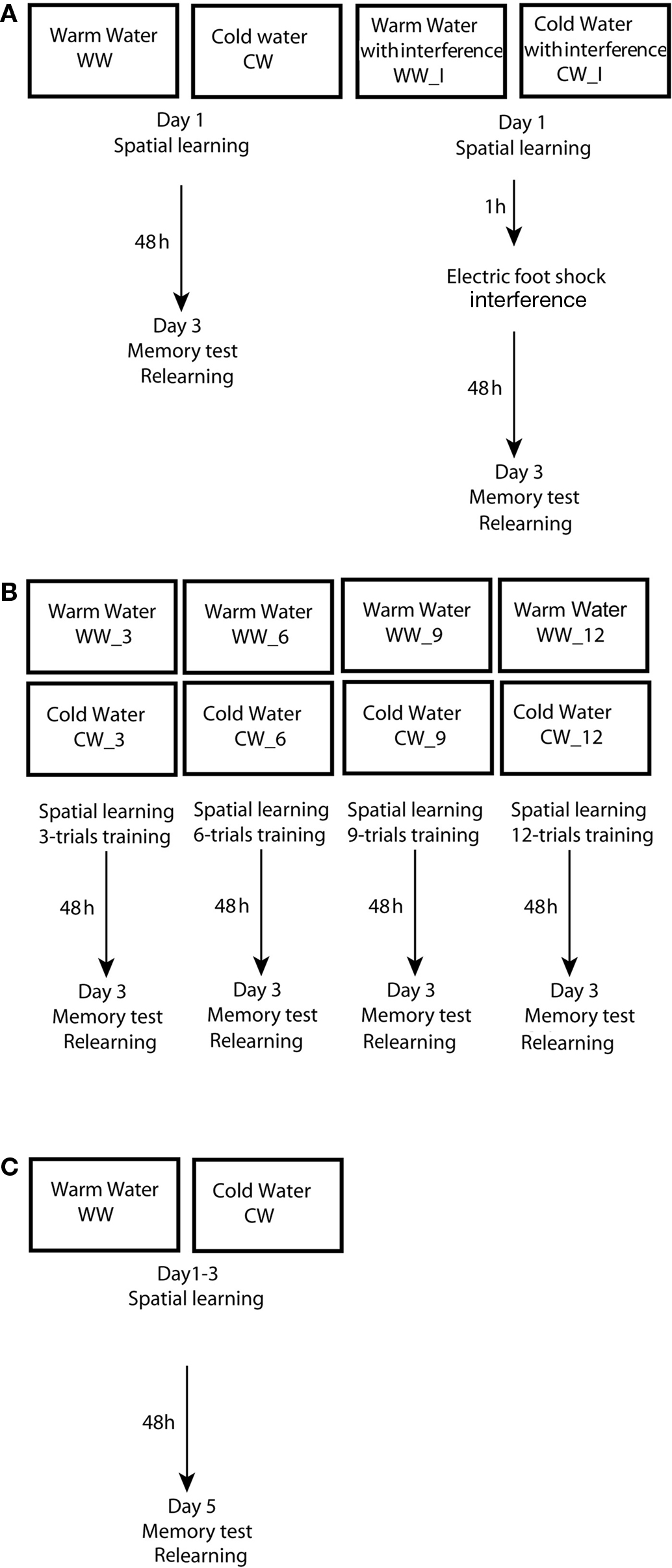
Figure 1. Experimental design used in the study. (A) Design of experiment 1 – spatial learning and memory formation in the Morris water maze under warm or cold water conditions, with an electrical foot shock interference; (B) Design of experiment 2 – acquisition threshold for spatial memory formation in the Morris water maze under warm or cold water conditions; (C) Design of experiment 3 – spatial learning and memory formation in the Morris water maze under warm or cold water conditions.
Experiment 2 – acquisition threshold for spatial memory formation
Animals were subjected to spatial training in the Morris water maze utilizing a massed protocol of training that consisted of either 3, 6, 9, or 12 trials, under WW or CW conditions (WW_3, n = 12 and CW_3, n = 10; WW_6, n = 10 and CW_6, n = 9; WW_9, n = 9 and CW_9, n = 10; WW_12, n = 14 and CW_12, n = 14). On the third day, retention was tested by a relearning session; the animals were introduced to two trials in the water with platform located in the original quadrant beneath the water surface. Inter-trial interval was 1 min (Figure 1B).
Experiment 3 – spaced training spatial memory formation
Animals were subjected to spatial training in the Morris water maze using a spaced training protocol that consisted of 12 trials; the animals were subjected to 4 trials per day for three consecutive days (Sandi et al., 1997) with inter-trial alternating intervals of 1 and 4 min (WW = 11; CW = 11). On the fifth day (48 h after the last day of training), retention was tested by a relearning session; the animals were introduced to two trials in the water maze with platform located in the original quadrant beneath the water surface. Inter-trial interval was 1 min (Figure 1C).
Statistical Analysis
The results are expressed as means ± SEM. For statistical analyses, Student’s t-test, ANOVA for repeated measures and two-way ANOVA were used. α for statistical tests was set at 0.05.
Results
Experiment 1 – the Effects of Interference During Consolidation on Spatial Memory Formation
Performance of the animals during acquisition of the spatial task showed a marginally significant difference between the acquisition rates of WW and CW animals (ANOVA for repeated measures, F(1,37) = 4, p = 0.052), however, no difference was found between the acquisition rates during the last four trials (t(37) = 1.32; N.S.; Figure 2A), indicating that these animals have eventually acquired the task to the same level.
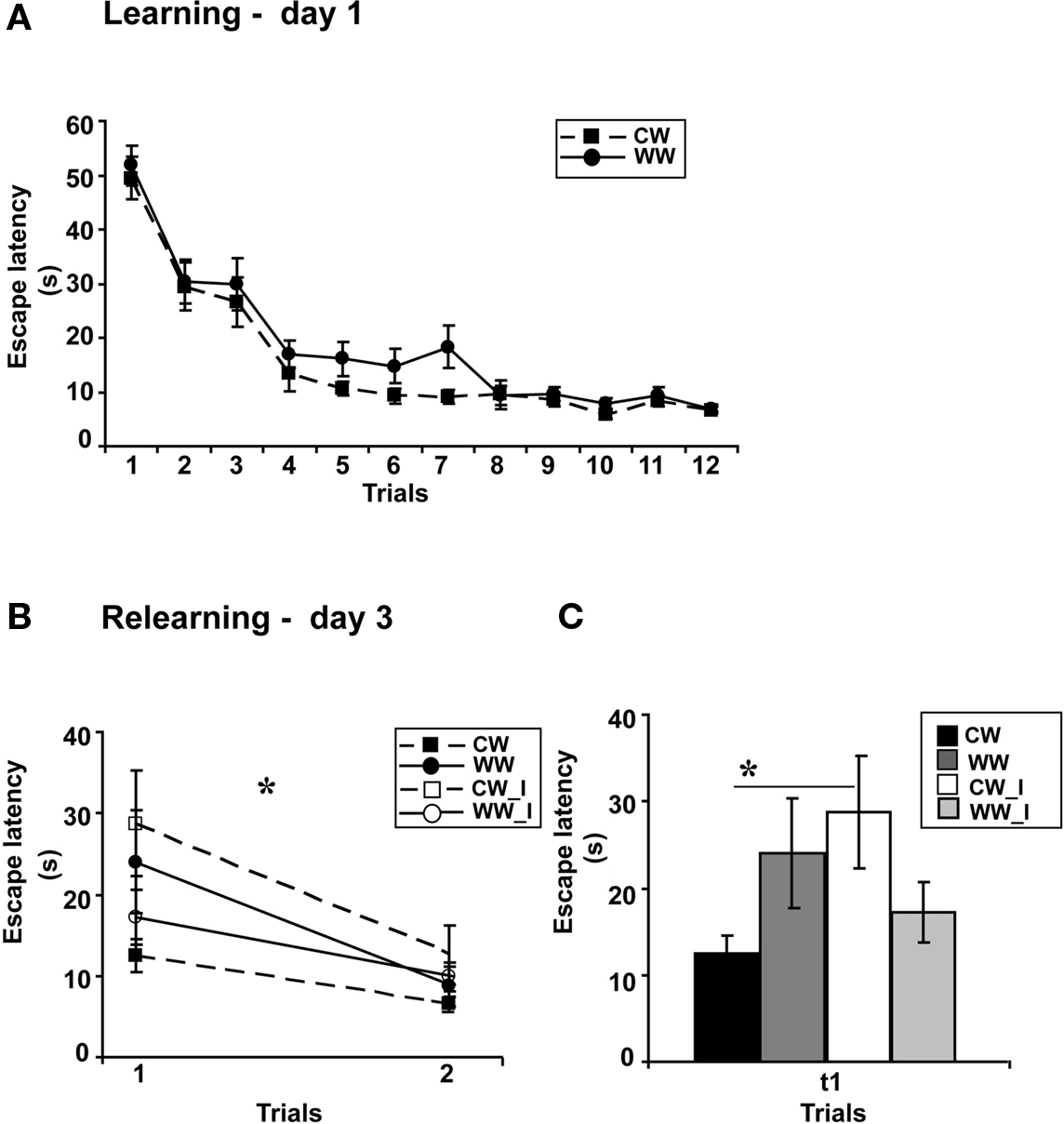
Figure 2. Spatial memory formed in the Morris water maze under different stress conditions, warm (24 ± 1°C) and cold (19 ± 1°C) water, when interfered by a foot shock stress. (A) Mean escape latencies during the 4 final trials of initial training did not differ between WW and CW animals; (B) The foot shock interference procedure impaired memory retention as tested on day 3 (during relearning) in CW animals, but not in WW animals; (C) The mean escape latency of the cold water animals following interference during the first trial was significantly longer than that of the cold water group that experienced no interference (*p < 0.05). (CW, Cold water; WW, Warm Water; CW_I, Cold Water with interference; WW_I, Warm Water with interference).
Two days later, examining retention following learning with or without shock interference under WW or CW conditions, two-way ANOVA analysis revealed a significant interaction effect between “water temperature” and “group” (F(1,35) = 5.1, p < 0.05). Further analysis indicated a difference between learning without and learning with interference under the CW condition (F(1,18) = 5, p < 0.05), with increased escape latency during the first trial of relearning, i.e., impaired memory retention, of the interference group as compared to the group that experienced no retrieval interference. The same interference protocol did not interrupt long-term memory generated under the WW condition (F(1,18) = 0.6, N.S.; Figures 2B,C).
Experiment 2 – Acquisition Threshold for Spatial Memory Formation
Performance during acquisition of the spatial task of the animals in this experiment was not different between the acquisition of the WW and CW animals trained for 12 trials (F(1,22) = 0.7, N.S.). Similarly, no difference in acquisition was found between the animals trained under the WW or CW conditions when animals were trained for 9 trials (ANOVA for repeated measures, F(1,17) = 0.00, N.S), for 6 trials (ANOVA for repeated measures, F(1,17) = 0.54, N.S) or for 3 trials (F(1,20) = 1.48, N.S.; Figure 3A).
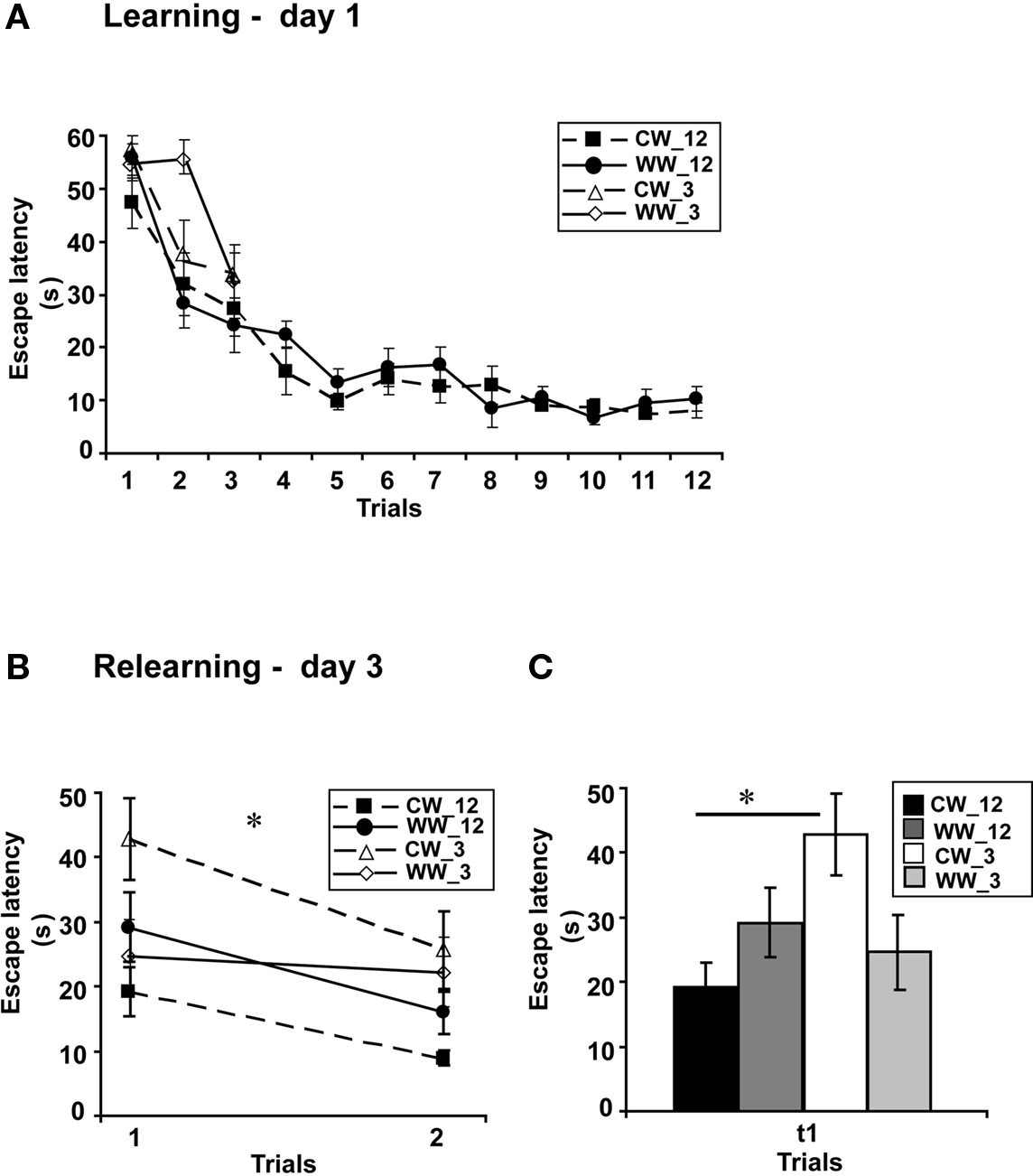
Figure 3. Spatial memory formed in the Morris water maze under different stress conditions, with 3-trials versus 12-trials training. (A) Mean escape latencies for each of 12 or 3 initial training trials (day 1) did not differ between WW and CW animals; (B) Memory retention tested on day 3 (during relearning) of the animals trained for 3 trials was impaired as compared to that of the animals trained for 12 trials in CW, but not in WW animals; (C) The mean escape latency of the cold water animals trained for 3 trials during the first trail was significantly longer than that of the cold water group trained for 12 trials (*p < 0.05). (CW_12, Cold Water 12-trials training; WW_12, Warm Water 12-trials training; CW_3, Cold Water 3-trials training; WW_3, Warm Water 3-trials training).
However, when long-term spatial memory generated following 3-trials, 6-trials, 9-trials, and 12-trials training under WW or CW was examined, an interaction effect between “water temperature” and “group” was found (two-way ANOVA, F(3,83) = 3.5, p < 0.05). Further analysis detected a significant difference between the groups of 3-trials training and 12-trials training under the CW condition (F(3,42) = 4.9, p < 0.05), with increased escape latency during the first trial of relearning (i.e., impaired retention) of the 3-trials training group relative to the 12-trials training group. In contrast, no such difference was found for the animals trained under the WW condition (F(3,41) = 0.6, N.S.; Figures 3B,C).
Experiment 3 – Spaced Training Spatial Memory Formation
Performance during acquisition of the spatial task was not different between the WW and CW groups (ANOVA for repeated measures, F(1,20) = 0.024, N.S.; Figure 4A).
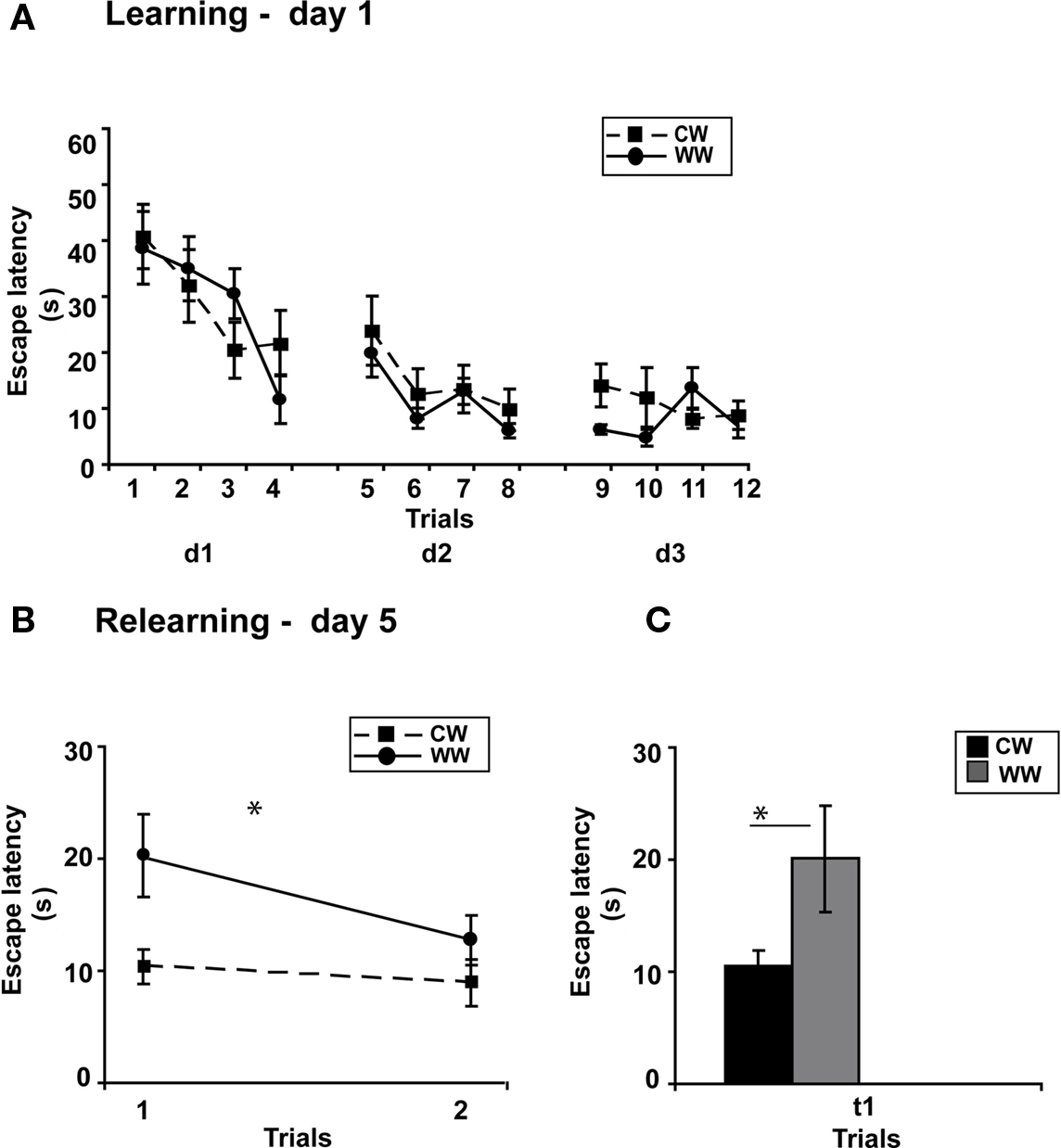
Figure 4. Spatial memory formed in the Morris water maze using spaced training under different stress conditions. (A) Mean escape latencies for each of 12 initial training trials (day 1–3) did not differ between WW and CW animals; (B) However, memory retention as tested on day 5 (during relearning) was better in CW animals compared to WW animals; (C) The mean escape latency of the warm water animals during the first trail was significantly longer than that of the cold water animals (*p < 0.05). (CW, Cold Water; WW, Warm Water).
Two days later, when memory performance was examined, retention of the CW group was significantly better compared to the WW group (ANOVA for repeated measures, F(1,20) = 6.230, p < 0.05), with decreased escape latency of the CW animals during the first trial of relearning (t(11) = 2.257, p < 0.05; Figures 4B,C).
Discussion
In recent years the notion that emotionality is associated with enhanced long-term memory has gained support from various lines of research. Human studies have demonstrated enhanced memory for emotional stimuli compared with neutral stimuli (Cahill and McGaugh, 1995; Cahill et al., 1996; Cahill et al., 2003; Quevedo et al., 2003); moreover, enhancement of long-term memory for neutral stimuli that was followed by emotional arousal was evidenced (Anderson et al., 2006). In addition, emotionally arousing information was found to activate the amygdala. Activity in the amygdala during encoding (Cahill et al., 1996; Hamann et al., 1999; Dolcos et al., 2004) and during retrieval (Dolcos et al., 2005) was more strongly correlated with emotional than with neutral information (Cahill et al., 1996), suggesting that the amygdala contributes to the memory-enhancing effects of emotionality.
Indeed, animal studies have demonstrated that intra-amygdala processes can enhance memory formation processes in other brain areas, such as the striatum (Packard et al., 1994), EC (Roesler et al., 2002) and hippocampus (Packard et al., 1994; Roozendaal and McGaugh, 1997; Kim et al., 2001). Furthermore, activation of the BLA was found to enhance neural plasticity in the hippocampus (Ikegaya et al., 1995; Akirav and Richter-Levin, 1999; Frey et al., 2001). These findings have led to the assumption that emotionality activates the amygdala, which then modulates memory consolidation processes in other brain areas (McGaugh et al., 1996; Richter-Levin, 2004).
We have previously demonstrated that training animals for a spatial learning task in the Morris water maze under CW, resulted in heightened activation of the BLA (as measured as activation of CREB or ERK2) compared with similar training under WW conditions (Akirav et al., 2001; Kogan and Richter-Levin, 2008). Since this heightened level of amygdala activation was found in animals with seemingly similar learning curves under both stress conditions, we hypothesized that the amygdala may contribute to other aspects of the memory formed, such as memory maintenance or its resistance to interference; we suggested that the memory trace would be stronger and more resistance to interference in the CW animals.
However, using a massed training protocol, and examining the impact of training rats for a spatial learning task in the water maze under either WW or CW conditions on these memory attributes, we have demonstrated that interference during the consolidation period following spatial training affected CW rather than WW group memory retention (Kogan and Richter-Levin, 2008). To further explore the effects of learning under WW versus CW conditions on spatial memory attributes, we have examined here first an additional interference procedure. In addition, the effects of altering levels of emotionality during acquisition on the threshold to acquire spatial memory and on memory formation following the more effective, spaced spatial training protocol were examined.
We have previously used as consolidation interference a reversal training protocol 1 h following the last training session (Kogan and Richter-Levin, 2008). Here we have repeated the interference experiment, but this time instead of reversal spatial training we have employed an electric foot shock as the interference procedure. This way, the interference effect could not be explained by a competition with a second spatial memory trace (the reversal location trace). The results of both the previous and current experiments indicate that the consolidation process under CW condition is more vulnerable to interference compared to consolidation under WW condition. Similar findings were reported by Wright and Conrad (2008). They found that spatial learning and memory in the water maze was impaired by prior exposure to stress only in animals trained under CW (19°C) but not under WW (24°C) conditions (Wright and Conrad, 2008).
It should be mentioned that in these series of experiments we have used different procedures of interference to the memory consolidation; each could serve as additional stressor and act to elevate corticosterone levels. However, several lines of research indicated that additional stress exerts a selective adverse effect on retrieval, rather than consolidation processes (Roozendaal, 2002). Different stressors, such as restraint, shock, and predator exposure, were reported to impair the spatial memory retention when administered 30 min before the 24 h memory test and not immediately after the acquisition phase (day before the memory test; de Quervain et al., 1998; Woodson et al., 2003).
We next examined the effects of altering levels of emotionality during acquisition on the threshold for acquiring efficient spatial memory. Memory retention of the 3-trials WW animals was not different from that found in the WW group that was trained for the full 12 trials during the first day. Three trials of training during the first day were not sufficient for the CW group and their performance on the third test day was lower compared to that of 12-trials animals, indicating that consolidation in these animals was less efficient than that in the WW group.
When maintenance of the spatial memory formed was examined (following complete training and without interference), a tendency was found in both experiments for the CW animals to exhibit better performance compared to the WW animals on the third, test day. This suggested the possibility that spatial memory consolidation under higher stress might involve more complex processing and thus renders it vulnerable to interference; but once formed, the memory trace may be stronger and its retention is more effective.
Multiple training trials are generally more effective for the production of robust learning and memory when they are spaced apart rather than when they are massed together. Spaced training enhances long-term memory by more effectively recruiting protein-synthesis-dependent mechanisms (Scharf et al., 2002). Several behavioral studies found improved consolidation of hippocampus-dependent memory following temporally spaced training and even overcoming learning deficits in the water maze subsequent to CREB inhibited activity (Kogan et al., 1997). In order to explore the possibility that higher stress conditions may indeed lead to a stronger spatial memory trace we conducted an additional experiment, utilizing a spaced training protocol (Sandi et al., 1997).
Under such conditions the previous tendency for better performance of the CW group became significant; suggesting that indeed, once established, the spatial memory formed under higher levels of stress is better maintained compared to spatial memory formed under lower levels of stress. This finding is consistent with results of Sandi et al. (1997), which also showed a quicker rate of acquisition and better long-term retention during spaced training under CW condition and not under WW condition.
Glucocorticoids (i.e., corticosterone in rats and cortisol in humans) were found to be major factor in emotionality and memory interactions (McGaugh and Roozendaal, 2002). More specifically, the role of corticosterone was extensively studied and emphasized with respect to stress influence on hippocampus-dependent learning and memory (Park et al., 2008). Inverted U-shape relationship between corticosterone levels and memory were described, with extremely low or high levels impairing memory and intermediate levels improving it (Conrad et al., 1999; Lupien et al., 1999; Sandi and Rose, 1997).
The U-shaped relationship between corticosterone and spatial memory following water maze training under different water temperatures was previously suggested (Sandi et al., 1997; Akirav et al., 2001, 2004).
However, series of experiments demonstrated that corticosterone injection without the accompanied stress did not affect spatial memory (Woodson et al., 2003; Park et al., 2008); therefore the emotional component of the experience is essential. Also, in humans, cortisol affected declarative memory only when it was accompanied with emotionally arousing information as opposed to the emotionally neutral information (Buchanan and Lovallo, 2001). It has also been shown that stress effects on hippocampal LTP and memory can be blocked without any reduction in the stress-evoked increase in corticosterone levels (Park et al., 2008). For example, suppression of amygdala functioning can block the effects of stress on spatial memory and hippocampal LTP without affecting the stress-induced increase in corticosterone levels (Kim et al., 2001, 2005). These findings indicate that elevated corticosterone levels alone may not be sufficient to impair memory.
Thus, the U-shaped function between corticosterone and memory is only one aspect in stress effects on memory. It is argued that fear-provoking conditions, which are known to engage the amygdala, interact with stress levels of corticosterone to influence hippocampal functioning (Roozendaal et al., 1996; Roozendaal and McGaugh, 1997; Cahill and McGaugh, 1998; Roozendaal et al., 1998; Kim et al., 2001; Kim and Diamond, 2002; McGaugh, 2004, 2006). Corticosterone effects on hippocampus-dependent memory were reported to be blocked by inactivation or lesioning of the amygdala (Kim et al., 2001, 2005; Roozendaal et al., 2002). Further, the glucocorticoid-induced enhancement of memory consolidation was found to require noradrenergic activation within the BLA, suggesting the synergistic actions of glucocorticoids and emotional arousal-induced noradrenergic activation of the BLA might lead to enhanced memory consolidation for emotionally arousing experiences (Roozendaal et al., 2006a,b).
The dual effect of emotionality may be explained by alterations in the relative contribution of brain areas involved in the process of consolidation (Adolphs et al., 1997; Hamann et al., 1999; Layton and Krikorian, 2002; Dolcos et al., 2004, 2005; Richardson et al., 2004; Smith et al., 2006). Previous studies have demonstrated that the shift from lower to higher emotionality is associated with an increased involvement of the amygdala and, probably, the DG of the hippocampus, but with a decreased involvement of the CA1 area of the hippocampus and, probably, also of the EC (Vouimba et al., 2004; Vouimba and Richter-Levin, 2005). Similarly, we have previously shown that while the dorsal CA1 is predominantly activated when training for a spatial task under WW condition, its relative involvement (as measured by CREB activation) is reduced when learning the same task under CW conditions. In contrast, the amygdala was found to be predominantly activated when training for a spatial task was conducted under the CW, i.e., higher stress conditions (Kogan and Richter-Levin, 2008). In a recent human study similar results concerning the role of the hippocampus under stress were obtained. Reduced hippocampal responses were associated with better declarative memory formation under stress when the learning task was fully embedded in a stressful context (Henckens et al., 2009). Such a declarative memory enhancement is evidently beneficial for survival, but the same mechanism may become maladaptive and culminate in mental diseases such as posttraumatic stress disorder (PTSD; Henckens et al., 2009).
It is possible that spatial learning under cold water with increased amygdala activation and reduced hippocampus involvement could represent one component of the PTSD, augmentation of memory for traumatic stimulus; but, when emotional memory consolidation is interfered, spatial memory impairments would appear. The results in the acquisition threshold experiment could also support this explanation, as under more stressful, CW, conditions, with decreased hippocampal activity, spatial learning consisting of 3-trials training was not sufficient for spatial memory formation compared to the full 12-trials training, while no such difference was found under the WW conditions with increased hippocampus activation.
These modifications in the pattern of activation of brain regions involved in learning and emotionality may underlie the alterations in the quality of memory attributes, such as memory maintenance and sensitivity to interference, described above.
Summary
We have previously shown that the protocols utilized here resulted in increased activation of the BLA, and have suggested that the amygdala may then support memory related processes in other brain areas (Akirav et al., 2001; Kogan and Richter-Levin, 2008). The current findings support this view, but demonstrate that the way emotionality enhances memory formation is somewhat more complex than was initially appreciated. Emotionality may activate the amygdala and the amygdala may support memory formation. However, this beneficial effect of emotionality has a price. Under heightened emotionality the process of consolidation seems to become more complex and thus vulnerable to interference.
The study demonstrates the possibility that different aspects of memory would be differentially affected by alterations in the level of emotionality. These differential effects on memory attributes may underlie the complex effects of stress and, particularly, of traumatic stress on memory, since the mixture of memory impairment together with augmented emotional memory is a key symptom in PTSD. Thus, unraveling the complexity of stress effects on memory formation and consolidation may contribute to our understanding of this disorder.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adolphs, R., Cahill, L., Schul, R., and Babinsky, R. (1997). Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn. Mem. 4, 291–300.
Akirav, I., Kozenicky, M., Tal, D., Sandi, C., Venero, C., and Richter-Levin, G. (2004). A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn. Mem. 11, 1887–1894.
Akirav, I., and Richter-Levin, G. (1999). Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J. neurosci. 19, 10530–10535.
Akirav, I., Sandi, C., and Richter-Levin G. (2001). Differential activation of hippocampus and amygdala following spatial learning under stress. Eur. J. Neurosci. 14, 1–8.
Anderson, A. K., Wais, P. E., and Gabrieli, J. D. (2006). Emotion enhances remembrance of neutral events past. Proc. Natl. Acad. Sci. U.S.A. 103, 1599–1604.
Buchanan, T. W., and Lovallo, W. R. (2001). Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 26, 307–317.
Cahill, L., Gorski, L., and Le, K. (2003). Enhanced human memory consolidation with post-learning stress, interaction with the degree of arousal at encoding. Learn. Mem. 10, 270–274.
Cahill, L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., Wu, J., and McGaugh, J. L. (1996). Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc. Natl. Acad. Sci. U.S.A. 93, 8016–8021.
Cahill, L., and McGaugh, J. L. (1995). A novel demonstration of enhanced memory associated with emotional arousal. Conscious. Cogn. 4, 410–421.
Cahill, L., and McGaugh, J. L. (1998). Emotional arousal and declarative memory. Trends Neurosci. 21, 294–299.
Conrad, C. D., Lupien, S. D., and McEwen, B. S. (1999). Support for bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol. Learn. Mem. 72, 39–46.
de Quervain, D. J., Roozendaal, B., and McGaugh, J. L. (1998). Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394, 787–790.
Dolcos, F., LaBar, K. S., and Cabeza, R. (2004). Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42, 855–863.
Dolcos, F., LaBar, K. S., and Cabeza, R. (2005). Remembering one year later, Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc. Natl. Acad. Sci. U.S.A. 102, 2626–2631.
Frey, S., Bergado-Rosado, J., Seidenbecher, T., Pape, H. C., and Frey, J. U. (2001). Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala, heterosynaptic induction mechanisms of late-LTP. J. Neurosci. 21, 3697–3703.
Hamann, S. B., Ely, T. D., Grafton, S. T., and Kilts, C. D. (1999). Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat. Neurosci. 2, 289–293.
Henckens, M. J., Hermans, E. J., Pu, Z., and Joëls, M., Fernández, G. (2009). Stressed memories: how acute stress affects memory formation in humans. J. Neurosci. 29, 10111–10119.
Ikegaya, Y., Saito, H., and Abe, K. (1995). High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci. Res. 22, 203–207.
Kim, J. J., and Diamond, D. M. (2002).The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 3, 453–462.
Kim, J. J., Koo, J. W., Lee, H. J., and Han, J. S. (2005). Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J. Neurosci. 25, 1532–1539.
Kim, J. J., Lee, J., Hans, J. S., and Packard, M. G. (2001). Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J. Neurosci. 21, 222–228.
Kogan, I., and Richter-Levin, G. (2008). Activation pattern of the limbic system following spatial learning under stress. Eur. J. Neurosci. 27, 715–722.
Kogan, J. H., Frankland, P. W., Blendy, J. A., Coblentz, J., Marowitz, Z., Schutz, G., and Silva, A. J. (1997). Spaced training induces normal long-term memory in CREB mutant mice. Curr. Biol. 7, 1–11.
Layton, B., and Krikorian, R. (2002). Memory mechanisms in posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci. 14, 254–261.
Lupien, S. J., Gillin, C. J., and Hauger, R. L. (1999). Working memory is more sensitive than declarative memory to the acute effects of corticoids, a dose-response study in humans. Behav. Neurosci. 113, 420–430.
McGaugh, J. L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27, 1–28.
McGaugh, J. L. (2006). Make mild moments memorable: add a little arousal. Trends Cogn. Sci. 10, 345–347.
McGaugh, J. L., Cahill, L., and Roozendaal, B. (1996). Involvement of the amygdala in memory storage, interaction with other brain systems. Proc. Natl. Acad. Sci. U.S.A. 93, 13508–13514.
McGaugh, J. L., and Roozendaal, B. (2002). Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 21, 205–210.
Morris, R. G. M., Garrud, P., Rawlins, J. N. P., and O’Keefe, J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683.
Quevedo, J., Sant’ Anna, M. K., Madruga, M., Lovato, I., de-Paris, F., Kapczinski, F., Izquierdo, I., and Cahill, L. (2003). Differential effects of emotional arousal in short- and long-term memory in healthy adults. Neurobiol. Learn. Mem. 79, 132–135.
Packard, M. G., Cahill, L., and McGaugh, J. L. (1994). Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc. Natl. Acad. Sci. U.S.A. 91, 8477–8481.
Park, C. R., Zoladz, P. R., Conrad, C. D., Fleshner, M., and Diamond, D. M. (2008). Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn. Mem. 15, 271–280.
Richardson, M. P., Strange, B. A., and Dolan, R. J. (2004). Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat. Neurosci. 7, 278–285.
Richter-Levin, G. (2004). The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist 10, 31–39.
Roesler, R., Roozendaal, B., and McGaugh, J. L. (2002). Basolateral amygdala lesions block the memory enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. Eur. J. Neurosci. 15, 905–910.
Roozendaal, B. (2002). Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 78, 578–595.
Roozendaal, B., Brunson, K. L., Holloway, B. L., McGaugh, J. L., and Baram, T. Z. (2002). Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 99, 13908–13913.
Roozendaal, B., and McGaugh, J. L. (1997). Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 9, 76–83.
Roozendaal, B., Okuda, S., Van der Zee, E. A., and McGaugh, J. L. (2006a). Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 103, 6741–6746.
Roozendaal, B., Hui, G. K., Hui, I. R., Berlau, D. J., McGaugh, J. L., and Weinberger, N. M. (2006b). Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol. Learn. Mem. 86, 249–255.
Roozendaal, B., Portillo-Marquez, G., and McGaugh, J. L. (1996). Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial memory. Behav. Neurosci. 110, 1074–1083.
Roozendaal, B., Sapolsky, R. M., and McGaugh, J. L. (1998). Basolateral amygdala lesions block the disruptive effects of long-term adrenalectomy on spatial memory. Neuroscience 84, 453–465.
Sandi, C., Loscertales, M., and Guaza, C. (1997). Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 9, 637–642.
Sandi, C., and Rose, S. P. (1997).Training-dependent biphasic effects of corticosterone in memory formation for a passive avoidance task in chicks. Psychopharmacology 133, 152–160.
Schaaf, M. J., Sibug, R. M., Duurland, R., Fluttert, M. F., Oitzl, M. S., De Kloet, E. R., and Vreugdenhil, E. (1999). Corticosterone effects on BDNF mRNA expression in the rat hippocampus during Morris water maze training. Stress 3, 173–183.
Scharf, M. T., Woo, N. H., Lattal, K. M., Young, J. Z., Nguyen, P. V., and Abel, T. (2002). Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J. Neurophysiol. 87, 2770–2777.
Smith, A. P. R., Stephan, K. E., Rugg, M. D., and Dolan, R. J. (2006). Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron 9, 631–638.
Squire, L. R., and Zola-Morgan, S. (1991). The medial temporal lobe memory system. Science 253, 1380–1386.
Vouimba, R. M., and Richter-Levin, G. (2005). Physiological dissociation in hippocampal subregions in response to amygdala stimulation. Cereb. Cortex 15, 1815–1821.
Vouimba, R. M., Yaniv, D., Diamond, D., and Richter-Levin, G. (2004). Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur. J. Neurosci. 19, 1887–1894.
Woodson, J. C., Macintosh, D., Fleshner, M., and Diamond, D. M. (2003). Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn. Mem. 10, 326–336.
Keywords: consolidation, emotional memory, interference
Citation: Kogan I and Richter-Levin G (2010) Emotional memory formation under lower versus higher stress conditions. Front. Behav. Neurosci. 4:183. doi: 10.3389/fnbeh.2010.00183
Received: 11 July 2010;
Paper pending published: 09 August 2010;
Accepted: 23 November 2010;
Published online: 06 December 2010
Edited by:
Regina M. Sullivan, Nathan Kline Institute and New York University School of Medicine, USAReviewed by:
Oliver Stork, National Institute for Physiological Sciences, JapanMarie H. Monfils, University of Texas at Austin, USA
Copyright: © 2010 Kogan and Richter-Levin. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Gal Richter-Levin, Departments of Psychology, Neurobiology and Ethology and the Brain and Behavior Research Center, University of Haifa, Haifa 31905, Israel. e-mail: gal.r-l@psy.haifa.ac.il