Lateral gene transfers have polished animal genomes: lessons from nematodes
- 1Institut National de la Recherche Agronomique, UMR 1355 ISA, 400 route des Chappes, Sophia-Antipolis, France
- 2Centre National de la Recherche Scientifique, UMR 7254 ISA, 400 route des Chappes, Sophia-Antipolis, France
- 3Université de Nice-Sophia Antipolis, UMR ISA, 400 route des Chappes, Sophia-Antipolis, France
It is now accepted that lateral gene transfers (LGT), have significantly contributed to the composition of bacterial genomes. The amplitude of the phenomenon is considered so high in prokaryotes that it challenges the traditional view of a binary hierarchical tree of life to correctly represent the evolutionary history of species. Given the plethora of transfers between prokaryotes, it is currently impossible to infer the last common ancestral gene set for any extant species. For this ensemble of reasons, it has been proposed that the Darwinian binary tree of life may be inappropriate to correctly reflect the actual relations between species, at least in prokaryotes. In contrast, the contribution of LGT to the composition of animal genomes is less documented. In the light of recent analyses that reported series of LGT events in nematodes, we discuss the importance of this phenomenon in the evolutionary history and in the current composition of an animal genome. Far from being neutral, it appears that besides having contributed to nematode genome contents, LGT have favored the emergence of important traits such as plant-parasitism.
Introduction
Lateral gene transfer (LGT), the transmission of genetic material from one species to another by means other than direct inheritance from parents to the offspring is a prevalent phenomenon in prokaryotes. LGT contribution to the composition of today bacterial genomes is so high that it challenges the idea that genome evolution in these species follows a tree-like pattern. Besides contributing to the composition of their genomes LGT events also play significant role in the acquisition of important biological traits. For instance, antibiotic resistance is frequently acquired during the lifespan of a bacterium and transmitted to its offspring rather than inherited from an ancient ancestor. Whether LGT events have significantly contributed to the composition of metazoan genomes and whether they are involved in the acquisition of new traits remains poorly documented, in contrast. This is probably in part because LGT in these species appears conceptually and mechanistically less evident than for prokaryotes. Here, we discuss an ensemble of LGT cases reported in different nematode lineages with different lifestyles, including necromenic, plant-parasitic, and animal-parasitic species. Although it is currently difficult to evaluate the contribution of LGT to the composition of nematode genomes, in the lack of a comprehensive whole genome scan, we can already assume that LGT have probably played important role in the acquisition of some traits such as plant-parasitism.
LGT in Necromenic Nematodes
The necromenic nematode Pristionchus pacificus spends a considerable part of its life cycle associated with beetles and is supposed to feed on bacteria and fungi that decompose the insect once it is dead. The genome sequencing of P. pacificus revealed the presence of functional cellulase genes acquired via LGT of microbial origin (Dieterich et al., 2008). A more detailed analysis of these cellulase genes in the Pristionchus genus showed that the genes were probably acquired ancestrally as different Pristionchus species possessed these genes and their phylogeny matched the species phylogeny (Mayer et al., 2011). The same analysis also showed that these genes underwent high rates of duplications and losses suggesting that the number of cellulase genes in Pristionchus species is under selection. Interestingly, the genome of P. pacificus also revealed the presence of Diapausin genes otherwise absent from nematodes but present in beetles. A phylogenetic analysis coupled with multi-species comparison of codon usage bias, revealed that Diapausin genes as well as ca. 500 others in the P. pacificus genome might have been acquired via LGT of insect origin (Rodelsperger and Sommer, 2011). Most of the putatively transferred genes of insect origin were retrotransposons, and it is hypothesized that these mobile elements might have played themselves a role of vector in the transfer of other genes. These first clear examples of putative transfers between two metazoan animals indicate that our current view of the amplitude of the LGT phenomenon in animals might be underestimated because most analyzes have been so far restricted to transfers of non-metazoan origin.
LGT in Animal-Parasitic Nematodes
The mutualistic symbiosis between alpha-proteobacteria Wolbachia and Onchocercids has for long stimulated scientific interest due to the possibility to control these causing agents of severe human diseases, such as river blindness and elephantiasis by antibacterial treatments. The interdependence between the symbiont and its host seems to involve the supply by the nematode of amino acids required for Wolbachia growth and the ability of the symbiont to complete the synthesis of compounds crucial to the nematode such as heme, riboflavin and nucleotides (Foster et al., 2005; Ghedin et al., 2007). Several LGTs from endosymbiont to the nematode genome have been described (Fenn et al., 2006; Dunning Hotopp et al., 2007; McNulty et al., 2010) and may have been facilitated by the close association of the bacteria to germline cells in the female stage (Ferri et al., 2011; Fischer et al., 2011). Until recently, it was generally assumed that the presence of the bacterial symbiont in Onchocercids could result from a single colonization event in an ancestor of the lineage and that the bacteria was secondarily lost in a few nematode species able to proliferate free from bacteria. The presence of transcriptionally active bacterial genes in the genomes of two distantly related Wolbachia-free Onchocercids species indicates that these species might have acquired bacterial genes before symbiont loss (McNulty et al., 2010). However, evidence for a recent Wolbachia capture in an Onchocercid species associated with the Japanese black bear modifies the current appraisal on the co-evolution of the endosymbiont and its host (Ferri et al., 2011). In addition, a screen for the presence of the bacteria in yet unexplored genera and species identified 63% of Onchocercids devoid of Wolbachia, notably the ancestral Oswaldofilariinae (Ferri et al., 2011). It will be most interesting to screen the genomes of these bacteria-free nematodes to search for traces of LGT and assess whether they lost their symbiont after acquisition of bacterial genes.
LGT in Plant-Parasitic Nematodes
The ability to feed on plants arose at least three times independently in the phylum Nematoda (Blaxter et al., 1998). How nematodes evolved toward plant-parasitism is a fascinating question. All phytonematodes feed from the cytoplasm of living plant cells. Ectoparasites perforate plant tissues with a protrusible stylet that reaches the cell layers they feed on whereas endoparasites penetrate plant tissues. Both ecto- and endoparasites, therefore, require active degradation or softening of the physical barrier formed by plant cell walls. The endoparasitic cyst and root-knot nematodes are among the most notorious phytonematodes, causing major damages to crops worldwide. The identification in cyst nematodes of genes encoding cellulases most similar to bacterial enzymes paved the way for the assumption that LGT could have participated in adaptation to plant-parasitism (Smant et al., 1998). Since then, several nematode genes encoding cell wall-degrading or -modifying enzymes were presented as candidates for LGT (Davis et al., 2000). The analysis of root-knot nematode genomes revealed an unprecedented repertoire of cell wall-degrading and -modifying enzymes in an animal that covered six different protein families able to degrade all major polysaccharides of cell walls, i.e., cellulose, hemicellulose and pectin (Abad et al., 2008; Opperman et al., 2008). Homologs for these nematode cell wall-degrading enzymes were searched in public databases, checked for significance and used for systematic robust phylogenetic reconstruction (Danchin et al., 2010). Two noticeable findings from this analysis were that those genes most likely originated from several independent LGT events and that in most cases the closest relatives were found in bacteria from the rhizosphere. Indeed, LGT events have been traced back at different nodes of the nematoda phylogeny. Some LGT events appear so far lineage-specific, for instance, GH28 polygalacturonases were only found to date in root-knot nematodes. In contrast, other genes such as those encoding GH5 cellulases or PL3 pectate lyases are found in multiple plant-parasitic nematode lineages, suggesting a more ancient acquisition in a common ancestor. Interestingly, several different bacterial species appear as potential donors for the different families of plant cell wall-degrading enzymes. For example, the closest relatives of root-knot nematode GH28s are found in Ralstonia solanacearum, a plant-pathogenic soil bacterium that shares plant hosts with these nematodes. On the other hand, nematode PL3 pectate lyases cluster with PL3s of Clavibacter michiganensis, another plant pathogen evolutionary distant from Ralstonia solanacearum. Overall, this analysis suggests that the ecology of the donor is probably more important than its phylogenetic position for a gene transfer to occur. This is consistent with similar conclusions drawn from the analysis of LGT events between bacteria of the human microbiome (Smillie et al., 2011). Another interesting feature of genes encoding cell wall-degrading enzymes in nematodes is their frequent organization in large multigene families. As many as 30 pectate lyases, 21 cellulases and 20 expansin-like genes were identified in the genome of M. incognita (Abad et al., 2008) and similar gene family expansions were observed in the genome of M. hapla (Opperman et al., 2008). Gene abundance in these families suggest that gene duplications have been under positive selection during the evolution of these plant-parasites.
Several experimental data further indicate that LGT could have participated to adaptation to plant-parasitism. First, transcripts for all cell wall-degrading enzymes have been localized in the esophageal glands of the nematodes (Rosso et al., 2012). These oesophageal glands are specialized cells in which proteins secreted during infection are produced. In line with this finding, the systematic presence of a secretion signal peptide on the predicted protein sequences suggested a role for the enzymes outside the nematode during parasitism. Second, cellulases, pectate lyases, polygalacturonases, and expansin-like proteins were enzymatically active, although examples of potential pseudogenization were identified in migratory nematodes (Bera-Maillet et al., 2000; Popeijus et al., 2000; Jaubert et al., 2002; Qin et al., 2004; Mitreva-Dautova et al., 2006; Haegeman et al., 2010). Immunolocalization studies showed the secretion of cellulases and pectate lyases in planta during migration of the juveniles and comforted a role in cell wall degradation or cell wall softening during host invasion (Wang et al., 1999; Doyle and Lambert, 2002; Vieira et al., 2011). Moreover, immunolocalizations in root-knot nematode females provided a hint for an additional role of cellulases during egg laying at the surface of the root (Vieira et al., 2011). Finally, the reduced virulence of nematodes after knock-down of cellulase genes established the role of these enzymes in the success of infection (reviewed in Rehman et al., 2009; Rosso et al., 2009; Haegeman et al., 2009a).
Besides the clear examples of genes encoding cell wall-modifying enzymes, a series of other genes have been acquired by LGT in phytonematodes and also probably contributed to the emergence and success of plant-parasitism in these species (reviewed in Haegeman et al., 2011). The processes in which the gene products are putatively involved include crucial mechanisms of the parasitic interaction, such as establishment of the nematode's feeding structure, modulation of plant defense pathways and processing of nutrients absorbed from the plant. As for genes encoding cell wall-degrading enzymes, the principal putative donor species for these genes are soil bacteria but for a few other genes fungal species appear as the most likely donors.
Concluding Remarks
This bunch of indications for LGT occurrences in nematodes adds to other recent findings on LGT in eukaryotes and modifies our viewpoint on the prevalence of gene transfers in animals (for reviews see Keeling and Palmer, 2008; Dunning Hotopp, 2011). In plant parasitic nematodes, several LGT events occurred at different time points of their evolution. In addition, the transferred genes seem to originate from different donor organisms. However, any conclusion on the multiplicity of potential donors should be considered with caution as our view on gene transfers frequency between prokaryotes is evolving rapidly. Identification of the exact donor bacteria may be complicated by several aspects. The true donor bacteria might not exist anymore or might not have been characterized at the sequence level. In these cases the next bacteria sharing the highest similarity with the studied gene might be considered as donor by default. Also, due to the high frequency of transfers between bacteria, we cannot exclude that the gene transferred from a bacterium to a nematode had itself been already transferred before from another bacteria. Indeed, genes transferred multiple times have been described in proteobacteria (Kloesges et al., 2011). The observed transfer might thus be a secondary event.
How could foreign genes be integrated into the genome of nematodes is certainly the main black box here. Gene transfers from the endosymbiont Wolbachia in Onchocercids can be facilitated by the presence of the bacteria in germ cells, though the exact genetic mechanism of transfer is unknown.
Noticeably, no symbiont has been identified so far in the root-knot and cyst nematodes mentioned above. Still, ancestors of phytonematodes could have held bacterial symbionts, as suggested by the presence of an endosymbiotic bacterium from the Wolbachia supergroup within the reproductive tract of Radopholus similis females, plant-parasitic nematodes that share a common ancestor with cyst- and root-knot nematodes (Haegeman et al., 2009b). However, in the case of genes encoding plant cell wall-degrading enzymes, an origin from an ancient Wolbachia-like symbiont appears highly unlikely because in no phylogenetic analysis was a Wolbachia or Wolbachia-like sequence identified as an homolog (Danchin et al., 2010).
The two insect parasitic nematodes Steinernema and Heterorhabditis provide another example of close association between nematodes and bacteria. Gamma-proteobacteria dwell in the gut of infective juveniles of these two nematodes and in rectal glands of Heterorhabditis females (Ciche and Ensign, 2003). The genome of these nematodes is not yet available and if new cases of LGT of bacterial origin were identified here, they could indicate the possibility of transfer to the germline cells without direct contact with the bacteria.
An alternative hypothesis for the origin of LGT events is the “you are what you eat” hypothesis first formulated in (Doolittle, 1998). In root-knot nematodes, four different groups of bacteria can be viewed as potential donors of plant cell wall-degrading enzyme genes. Interestingly, three of these groups include notorious plant pathogens or symbionts having interactions within plant hosts and thereby sympatric with these nematodes (Danchin et al., 2010). One possibility is that an ancestor of plant-parasitic nematodes was initially bacterivorous and used to feed on soil bacteria, including plant-pathogenic bacteria (Figure 1). By mechanisms discussed later, genetic material from digested bacteria might have been transferred to the nuclear genome of nematodes. In the case of cell wall-degrading enzymes, the transferred genes likely provided the nematode the ability to penetrate plant tissue and in a first time to access root-dwelling bacteria otherwise inaccessible to other bacterivorous nematodes. This selective advantage might have favored individuals that possessed bacterial genes encoding cell wall-degrading enzymes and following a LGT ratchet mechanism, individuals with additional enzymes acquired via LGT might have been positively selected generation after generation and eventually developed a plant-parasitic lifestyle.
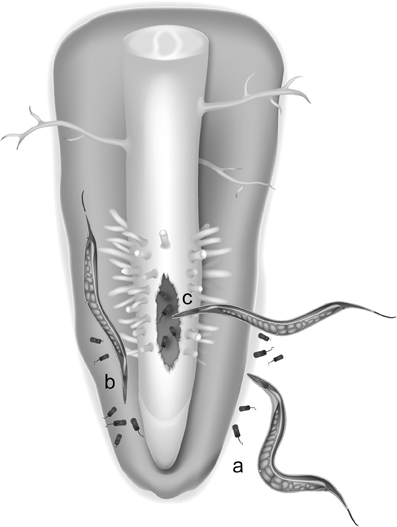
Figure 1. An hypothesis for lateral gene transfer from bacteria to plant-parasitic nematodes. LGT could have been favored in ancestral nematodes that shared their ecological niche with plant-associated bacteria in soil (a), in the rhizosphere (b) or in bacteria-infected plant tissues (c).
Whatever the origin, from ancient endosymbionts or from feeding, different potential mechanisms of transfer can be envisioned. They may involve bacterial secretion systems, bacteriophages, plasmids, or viruses as well as passive or active spread of DNA fragments from digested bacterial cells (Danchin, 2011). Transposable elements can also be considered as potential vectors, particularly in the case of Pristionchus in which retrostransposons have been found associated with transferred genes. In contrast, no evidence for a proximity of transposable elements in the vicinity of transferred genes in plant-parasitic nematodes has been described.
Overall, it appears that LGT, both from bacterial and fungal origins have not only contributed to the current composition of nematode gene repertoires but also had crucial importance in the emergence of new biological capabilities. Although several examples of LGT of non-metazoan origin in nematode genomes have been reported, the total contribution of LGT to the composition of a nematode genome is yet poorly described. To date, no comprehensive list of genes putatively acquired via LGT in a nematode genome has been published. It is also largely unknown whether transfers of animal or plant origin substantially contributed to the composition of nematode genomes. The recent example of transfers from insects to necromenic nematodes suggests this might actually be the case.
The idea that a Darwinian binary tree-like representation incorrectly reflects the evolutionary history of genes and genomes has been raised as soon as 1975 (Sneath, 1975). Based on the observation that LGT occur frequently in bacteria and that reticulate evolution, another phenomenon challenging binary tree-like representation is common in flowering plants, Sneath already proposed that a network-like representation might be more accurate. This idea has found echoes more recently in the light of whole genome analysis, suggesting that, at least in bacteria, evolution more resembles a rhizome than a bifurcating tree (Raoult, 2010). We cannot state at the moment to what extent could LGT events disturb a binary tree-like representation in the phylum nematoda. Regardless the potential contribution of LGT to the composition of nematode genomes, it appears clear, at least in the case of plant-parasitic nematodes that these events have contributed to adaptation to a new life style.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work benefited from links funded through European COST Action 872.
References
Abad, P., Gouzy, J., Aury, J. M., Castagnone-Sereno, P., Danchin, E. G., Deleury, E., Perfus-Barbeoch, L., Anthouard, V., Artiguenave, F., Blok, V. C., Caillaud, M. C., Coutinho, P. M., Dasilva, C., De Luca, F., Deau, F., Esquibet, M., Flutre, T., Goldstone, J. V., Hamamouch, N., Hewezi, T., Jaillon, O., Jubin, C., Leonetti, P., Magliano, M., Maier, T. R., Markov, G. V., McVeigh, P., Pesole, G., Poulain, J., Robinson-Rechavi, M., Sallet, E., Segurens, B., Steinbach, D., Tytgat, T., Ugarte, E., Van Ghelder, C., Veronico, P., Baum, T. J., Blaxter, M., Bleve-Zacheo, T., Davis, E. L., Ewbank, J. J., Favery, B., Grenier, E., Henrissat, B., Jones, J. T., Laudet, V., Maule, A. G., Quesneville, H., Rosso, M. N., Schiex, T., Smant, G., Weissenbach, J., and Wincker, P. (2008). Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 26, 909–915.
Bera-Maillet, C., Arthaud, L., Abad, P., and Rosso, M. N. (2000). Biochemical characterization of MI-ENG1, a family 5 endoglucanase secreted by the root-knot nematode Meloidogyne incognita. Eur. J. Biochem. 267, 3255–3263.
Blaxter, M. L., De Ley, P., Garey, J. R., Liu, L. X., Scheldeman, P., Vierstraete, A., Vanfleteren, J. R., Mackey, L. Y., Dorris, M., Frisse, L. M., Vida, J. T., and Thomas, W. K. (1998). A molecular evolutionary framework for the phylum Nematoda. Nature 392, 71–75.
Ciche, T. A., and Ensign, J. C. (2003). For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl. Environ. Microbiol. 69, 1890–1897.
Danchin, É. G. J. (2011). What Nematode genomes tell us about the importance of horizontal gene transfers in the evolutionary history of animals. Mobile Genet. Elem. 1, 1–5.
Danchin, E. G., Rosso, M. N., Vieira, P., De Almeida-Engler, J., Coutinho, P. M., Henrissat, B., and Abad, P. (2010). Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc. Natl. Acad. Sci. U.S.A. 107, 17651–17656.
Davis, E. L., Hussey, R. S., Baum, T. J., Bakker, J., Schots, A., Rosso, M. N., and Abad, P. (2000). Nematode parasitism genes. Annu. Rev. Phytopathol. 38, 365–396.
Dieterich, C., Clifton, S. W., Schuster, L. N., Chinwalla, A., Delehaunty, K., Dinkelacker, I., Fulton, L., Fulton, R., Godfrey, J., Minx, P., Mitreva, M., Roeseler, W., Tian, H., Witte, H., Yang, S. P., Wilson, R. K., and Sommer, R. J. (2008). The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat. Genet. 40, 1193–1198.
Doolittle, W. F. (1998). You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends Genet. 14, 307–311.
Doyle, E. A., and Lambert, K. N. (2002). Cloning and characterization of an esophageal-gland-specific pectate lyase from the root-knot nematode Meloidogyne javanica. Mol. Plant Microbe Interact. 15, 549–556.
Dunning Hotopp, J. C. (2011). Horizontal gene transfer between bacteria and animals. Trends Genet. 27, 157–163.
Dunning Hotopp, J. C., Clark, M. E., Oliveira, D. C., Foster, J. M., Fischer, P., Torres, M. C., Giebel, J. D., Kumar, N., Ishmael, N., Wang, S., Ingram, J., Nene, R. V., Shepard, J., Tomkins, J., Richards, S., Spiro, D. J., Ghedin, E., Slatko, B. E., Tettelin, H., and Werren, J. H. (2007). Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 317, 1753–1756.
Fenn, K., Conlon, C., Jones, M., Quail, M. A., Holroyd, N. E., Parkhill, J., and Blaxter, M. (2006). Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathog. 2:e94. doi: 10.1371/journal.ppat.0020094
Ferri, E., Bain, O., Barbuto, M., Martin, C., Lo, N., Uni, S., Landmann, F., Baccei, S. G., Guerrero, R., De Souza Lima, S., Bandi, C., Wanji, S., Diagne, M., and Casiraghi, M. (2011). New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS One 6:e20843. doi: 10.1371/journal.pone.0020843
Fischer, K., Beatty, W. L., Jiang, D., Weil, G. J., and Fischer, P. U. (2011). Tissue and stage-specific distribution of Wolbachia in Brugia malayi. PLoS Negl. Trop. Dis. 5:e1174. doi: 10.1371/journal.pntd.0001174
Foster, J., Ganatra, M., Kamal, I., Ware, J., Makarova, K., Ivanova, N., Bhattacharyya, A., Kapatral, V., Kumar, S., Posfai, J., Vincze, T., Ingram, J., Moran, L., Lapidus, A., Omelchenko, M., Kyrpides, N., Ghedin, E., Wang, S., Goltsman, E., Joukov, V., Ostrovskaya, O., Tsukerman, K., Mazur, M., Comb, D., Koonin, E., and Slatko, B. (2005). The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 3:e121. doi: 10.1371/journal.pbio.0030121
Ghedin, E., Wang, S., Spiro, D., Caler, E., Zhao, Q., Crabtree, J., Allen, J. E., Delcher, A. L., Guiliano, D. B., Miranda-Saavedra, D., Angiuoli, S. V., Creasy, T., Amedeo, P., Haas, B., El-Sayed, N. M., Wortman, J. R., Feldblyum, T., Tallon, L., Schatz, M., Shumway, M., Koo, H., Salzberg, S. L., Schobel, S., Pertea, M., Pop, M., White, O., Barton, G. J., Carlow, C. K., Crawford, M. J., Daub, J., Dimmic, M. W., Estes, C. F., Foster, J. M., Ganatra, M., Gregory, W. F., Johnson, N. M., Jin, J., Komuniecki, R., Korf, I., Kumar, S., Laney, S., Li, B. W., Li, W., Lindblom, T. H., Lustigman, S., Ma, D., Maina, C. V., Martin, D. M., McCarter, J. P., McReynolds, L., Mitreva, M., Nutman, T. B., Parkinson, J., Peregrin-Alvarez, J. M., Poole, C., Ren, Q., Saunders, L., Sluder, A. E., Smith, K., Stanke, M., Unnasch, T. R., Ware, J., Wei, A. D., Weil, G., Williams, D. J., Zhang, Y., Williams, S. A., Fraser-Liggett, C., Slatko, B., Blaxter, M. L., and Scott, A. L. (2007). Draft genome of the filarial nematode parasite Brugia malayi. Science 317, 1756–1760.
Haegeman, A., Jones, J. T., and Danchin, E. G. (2011). Horizontal gene transfer in nematodes: a catalyst for plant parasitism? Mol. Plant Microbe Interact. 24, 879–887.
Haegeman, A., Kyndt, T., and Gheysen, G. (2010). The role of pseudo-endoglucanases in the evolution of nematode cell wall-modifying proteins. J. Mol. Evol. 70, 441–452.
Haegeman, A., Vanholme, B., and Gheysen, G. (2009a). Characterization of a putative endoxylanase in the migratory plant-parasitic nematode Radopholus similis. Mol. Plant Pathol. 10, 389–401.
Haegeman, A., Vanholme, B., Jacob, J., Vandekerckhove, T. T., Claeys, M., Borgonie, G., and Gheysen, G. (2009b). An endosymbiotic bacterium in a plant-parasitic nematode: member of a new Wolbachia supergroup. Int. J. Parasitol. 39, 1045–1054.
Jaubert, S., Laffaire, J. B., Abad, P., and Rosso, M. N. (2002). A polygalacturonase of animal origin isolated from the root-knot nematode Meloidogyne incognita. FEBS Lett. 522, 109–112.
Keeling, P. J., and Palmer, J. D. (2008). Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 9, 605–618.
Kloesges, T., Popa, O., Martin, W., and Dagan, T. (2011). Networks of gene sharing among 329 proteobacterial genomes reveal differences in lateral gene transfer frequency at different phylogenetic depths. Mol. Biol. Evol. 28, 1057–1074.
Mayer, W. E., Schuster, L. N., Bartelmes, G., Dieterich, C., and Sommer, R. J. (2011). Horizontal gene transfer of microbial cellulases into nematode genomes is associated with functional assimilation and gene turnover. BMC Evol. Biol. 11, 13.
McNulty, S. N., Foster, J. M., Mitreva, M., Dunning Hotopp, J. C., Martin, J., Fischer, K., Wu, B., Davis, P. J., Kumar, S., Brattig, N. W., Slatko, B. E., Weil, G. J., and Fischer, P. U. (2010). Endosymbiont DNA in endobacteria-free filarial nematodes indicates ancient horizontal genetic transfer. PLoS One 5:e11029. doi: s10.1371/journal.pone.0011029
Mitreva-Dautova, M., Roze, E., Overmars, H., De Graaff, L., Schots, A., Helder, J., Goverse, A., Bakker, J., and Smant, G. (2006). A symbiont-independent endo-1,4-beta-xylanase from the plant-parasitic nematode Meloidogyne incognita. Mol. Plant Microbe Interact. 19, 521–529.
Opperman, C. H., Bird, D. M., Williamson, V. M., Rokhsar, D. S., Burke, M., Cohn, J., Cromer, J., Diener, S., Gajan, J., Graham, S., Houfek, T. D., Liu, Q., Mitros, T., Schaff, J., Schaffer, R., Scholl, E., Sosinski, B. R., Thomas, V. P., and Windham, E. (2008). Sequence and genetic map of Meloidogyne hapla: a compact nematode genome for plant parasitism. Proc. Natl. Acad. Sci. U.S.A. 105, 14802–14807.
Popeijus, H., Overmars, H., Jones, J., Blok, V., Goverse, A., Helder, J., Schots, A., Bakker, J., and Smant, G. (2000). Degradation of plant cell walls by a nematode. Nature 406, 36–37.
Qin, L., Kudla, U., Roze, E. H., Goverse, A., Popeijus, H., Nieuwland, J., Overmars, H., Jones, J. T., Schots, A., Smant, G., Bakker, J., and Helder, J. (2004). Plant degradation: a nematode expansin acting on plants. Nature 427, 30.
Rehman, S., Butterbach, P., Popeijus, H., Overmars, H., Davis, E. L., Jones, J. T., Goverse, A., Bakker, J., and Smant, G. (2009). Identification and characterization of the most abundant cellulases in stylet secretions from Globodera rostochiensis. Phytopathology 99, 194–202.
Rodelsperger, C., and Sommer, R. J. (2011). Computational archaeology of the Pristionchus pacificus genome reveals evidence of horizontal gene transfers from insects. BMC Evol. Biol. 11, 239.
Rosso, M. N., Hussey, R. S., Davis, E. L., Smant, G., Baum, T., Abad, P., and Mitchum, M. (2012). “Nematode effector proteins: targets and functions in plant parasitism,” in Effectors in Plant-Microbe Interactions, eds F. Martin and S. Kammoun (Chichester, UK: Wiley-Blackwell), 327–354.
Rosso, M. N., Jones, J. T., and Abad, P. (2009). RNAi and functional genomics in plant parasitic nematodes. Annu. Rev. Phytopathol. 47, 207–232.
Smant, G., Stokkermans, J. P., Yan, Y., De Boer, J. M., Baum, T. J., Wang, X., Hussey, R. S., Gommers, F. J., Henrissat, B., Davis, E. L., Helder, J., Schots, A., and Bakker, J. (1998). Endogenous cellulases in animals: isolation of beta-1,4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc. Natl. Acad. Sci. U.S.A. 95, 4906–4911.
Smillie, C. S., Smith, M. B., Friedman, J., Cordero, O. X., David, L. A., and Alm, E. J. (2011). Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480, 241–244.
Vieira, P., Danchin, E. G., Neveu, C., Crozat, C., Jaubert, S., Hussey, R. S., Engler, G., Abad, P., De Almeida-Engler, J., Castagnone-Sereno, P., and Rosso, M. N. (2011). The plant apoplasm is an important recipient compartment for nematode secreted proteins. J. Exp. Bot. 62, 1241–1253.
Keywords: lateral gene transfer, horizontal gene transfer, genome, nematode, eukaryote, adaptation, evolution
Citation: Danchin EGJ and Rosso M-N (2012) Lateral gene transfers have polished animal genomes: lessons from nematodes. Front. Cell. Inf. Microbio. 2:27. doi: 10.3389/fcimb.2012.00027
Received: 23 January 2012; Paper pending published: 14 February 2012;
Accepted: 21 February 2012; Published online: 06 March 2012.
Edited by:
Didier Raoult, Université de la Méditerranée, FranceReviewed by:
Kalliopi Georgiades, Unité de Recherche en maladies infectieuses tropicales emergentes, FrancePierre Pontarotti, Centre National de la Recherche Scientifique, France
Copyright: © 2012 Danchin and Rosso. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Etienne G. J. Danchin, Institut Sophia Agrobiotech, INRA-CNRS-UNSA, 400 route des Chappes, F-06903 Sophia-Antipolis, France. e-mail: etienne.danchin@sophia.inra.fr
†Present address: INRA, Université Aix-Marseille, UMR1163 Biotechnologie des Champignons Filamenteux, F-13288 Marseille, France.