1
Department of Experimental Psychology, University of Magdeburg, Magdeburg, Germany
2
Center for Behavioral Brain Sciences, Magdeburg, Germany
Anterior prefrontal cortex is usually associated with high level executive functions. Here, we show that the frontal pole, specifically left lateral frontopolar cortex, is involved in signaling change in implicitly learned spatial contexts, in the absence of conscious change detection. In a variant of the contextual cueing paradigm, participants first learned contingencies between distractor contexts and target locations implicitly. After learning, repeated distractor contexts were paired with new target locations. Left lateral frontopolar [Brodmann area (BA) 10] and superior frontal (BA9) cortices showed selective signal increase for this target location change in repeated displays in an event-related fMRI experiment, which was most pronounced in participants with high contextual facilitation before the change. The data support the view that left lateral frontopolar cortex is involved in signaling contextual change to posterior brain areas as a precondition for adaptive changes of attentional resource allocation. This signaling occurs in the absence of awareness of learned contingencies or contextual change.
Prefrontal cortex is almost synonymous with executive function. Especially the most anterior part of prefrontal cortex, roughly equivalent with Brodmann area (BA) 10, has been associated with high level cognitive processes like internal thought (Christoff and Gabrieli, 2000
), changing between externally driven and internal mental processes (Burgess et al., 2005)
, integration of several mental processes (Christoff et al., 2001
; Ramnani and Owen, 2004
) or hierarchical processing of several tasks (Braver and Bongiolatti, 2002
; Koechlin and Hyafil, 2007
), to name but a few.
In past experiments, however, we have found left lateral frontopolar activation in visual singleton feature search tasks, in which highly salient ‘odd-one out’ targets were to be detected (Pollmann et al., 2000
; Weidner et al., 2002
). Left lateral frontopolar cortex showed transient signal increases when the target defining feature dimension (e.g., color or movement) changed, along with increased search times in these dimension change trials compared with trials in which the target-defining dimension remained the same. Because of the apparently absent or very low demands on executive processes, these findings are not easily explained by the above-named hypotheses on anterior prefrontal function.
Behavioral (Müller et al., 1995
; Found and Müller, 1996
), neuroimaging (Pollmann et al., 2006
) and electrophysiological (Gramann et al., 2007
; Töllner et al., 2008
) evidence supports the view that dimension changes in visual singleton search lead to a shift of attentional weight from the old to the new target-defining dimension. A patient study demonstrated that lesions of left lateral anterior prefrontal cortex lead to selectively increased dimension change costs (Pollmann et al., 2007
). Taken together, the evidence suggests that left lateral anterior prefrontal cortex supports shifts of attention between visual dimensions (see also Rogers et al., 2000
).
While these data support an involvement of left lateral frontopolar cortex in the control of visual attention, it is as yet unknown what this role is. Attention changes usually involve not anterior prefrontal cortex, but the more posterior frontal and supplementary eye fields as the frontal components of a fronto-parietal network (Corbetta et al., 1998
; Gitelman et al., 1999
; Pollmann and von Cramon, 2000
; Corbetta and Shulman, 2002
). One hint at the role of frontopolar cortex comes from the finding that this area is selectively active during exogenously driven attention changes (Weidner et al., 2002
). Another hint comes from the observation that left lateral frontopolar cortex was activated in invalidly cued trials of an exogenous spatial cueing experiment (Lepsien and Pollmann, 2002
). This suggested that frontopolar cortex may be involved rather in reorienting attention from a currently attended focus than in attention shifts per se. This view is also in line with the increased search times in dimension-change trials in patients with lateral anterior prefrontal lesions (Pollmann et al., 2007
). Other hints come from memory research. Meta-analyses have shown that anterior prefrontal cortex, and specifically lateral frontopolar cortex, is consistently active during episodic retrieval (Christoff and Gabrieli, 2000
; Gilbert et al., 2006
). Left lateral prefrontal cortex was specifically involved when two retrieved episodic memory items needed to be compared (Reynolds et al., 2006
). Especially intriguing is the finding that lateral frontopolar cortex is sensitive to contextual interference (King et al., 2005
). In a memory study using virtual reality displays, King and colleagues found increased frontopolar activation at test when objects were associated to one of two locations and one of two persons in the study phase, compared to a version where each object was associated with a unique location and person.
Frontopolar Involvement in Implicit Change Processing
Based on these findings, we propose that frontopolar cortex may compare the current environment (in the lab experiment, e.g., a motion-defined target in a search display) with similar environments in the past (e.g., a color-defined target in the same display) to signal changes to structures of the fronto-parietal attention network (Corbetta et al., 1998
; Gitelman et al., 1999
; Pollmann and von Cramon, 2000
; Corbetta and Shulman, 2002
), thereby enabling a reallocation of attentional resources (in the example from color to movement) which is optimal for the new situation. Thus, episodic memory traces are compared to the current input to detect changes which may be relevant for an optimal allocation of attention. These changes are then signaled to other brain structures, which then initiate the attention changes. A candidate structure to receive input from frontopolar cortex (via intermediary neurons, as there are no direct fiber connections) may be the cortex at the temporo-parietal junction (TPJ), which is known to mediate disengagement from the current focus of attention to enable reallocation of attention to new aspects of the environment (Friedrich et al., 1998
; Corbetta et al., 2000
). While TPJ may not need input from frontopolar cortex when salient events command a reorienting of attention, frontopolar change detection signals may reach TPJ in cases when subtle, not consciously available changes occur, as in the present study.
Taken together, these hints from diverse sources led to our hypothesis that lateral frontopolar cortex may play an important role in attention control as an episodic monitoring instance which detects attention-relevant changes in familiar contexts, thereby enabling an optimization of attention allocation (Pollmann, 2004
). This optimization of attention is thought to proceed in an implicit, automatic manner, in the absence of consciousness. The latter assumption is backed by findings from several areas. Change-related frontopolar activation has been observed in singleton search, where changes of the target are logically irrelevant for task execution, because the task is to detect the ‘odd one out’ item, but not to identify it. In contrast, left lateral frontopolar activation has typically not been observed in tasks in which there is a clear rule-guided association between stimulus and response, e.g., in task switching studies (e.g., Dove et al., 2000
; Kimberg et al., 2000
; Sohn et al., 2000
; Ruge et al., 2003
). There is anecdotal evidence that in our singleton search experiments, which led to change-related anterior prefrontal activation, participants are not always aware of the nature of the singleton, even though they correctly detected it. A more direct demonstration of anterior prefrontal activation with unaware stimulus changes was reported by Konishi et al. (2003
). In their version of the Wisconsin Card Sorting Task, participants were cued when changes of the response-defining dimension occurred. However, unbeknownst to them, the feature values of the previous relevant dimension continued to indicate the correct response for a variable number of trials (i.e., the response was redundantly defined by the old and new dimension). The anterior prefrontal activation was observed only when the old dimension ceased to indicate the correct response. Taken together, this led us to propose that the proposed episodic change monitoring in frontopolar cortex may occur in the absence of conscious awareness (Pollmann, 2004
).
The Current Experiment
In order to test this hypothesis, we needed an experimental paradigm which would allow us to test implicit contextual change detection processes. We adapted the contextual cueing paradigm (Chun and Jiang, 1998
) in a way that enabled us to do just this. In contextual cueing, visual search is facilitated when the spatial distractor configuration in search displays is repeated. This learning of contextual cues is implicit, it may occur in the absence of explicit recollection of the repeated displays. In order to investigate implicit change detection processes, we adapted the contextual cueing paradigm by changing the target location in repeated displays after an initial learning phase. Response time and eye movement measurements have shown that the search advantage due to contextual cueing is lost after the change in target location in new displays and that subjects then relearn the new spatial configuration of target and surrounding distractors, all in the absence of awareness (Manginelli and Pollmann, 2009
).
Derived from our hypothesis of left lateral frontopolar cortex as an implicit contextual change detector, we predicted left lateral frontopolar activation following a target location change in displays with repeated distractor configuration. Recently, we reported a first confirmation of this hypothesis in that anterior prefrontal activation occurred when the target location changed in a repeated distractor context which was only repeated six times before the change (Pollmann and Manginelli, 2009
). This was an initial demonstration that anterior prefrontal cortex is sensitive to changes in the target–distractor configuration which was not consciously perceived. However, the few learning trials did not lead to a significant facilitation of search in the repeated displays. Thus, no conclusions could be drawn on the relation of anterior frontal change detection and visual learning. This is one main aspect of the current study. Therefore, the present experiment was designed to reach optimal learning of target–distractor contexts before the change of the target location occurred. For this purpose, participants took part in a full session of contextual cueing the day before the fMRI experiment. In the first half of the fMRI-experiment, they saw the same repeated displays as the day before, whereas in the second half, the displays with repeated distractor configurations contained the target at a new location.
If frontopolar cortex is involved in ‘detecting’ changes of implicitly learned contingencies, it should pass further tests. Frontopolar change-related activation should be strong if strong search facilitation, indicative of robust contextual learning, was observed prior to the change. Moreover, if frontopolar activation is due to a specific process of change detection, it should not be correlated with the search time costs induced by the target location change in old displays. Change detection should be of comparable duration across change trials, whereas search time varies considerably from trial to trial depending on variations of the search path and the (unknown) location of the target. Thus, a correlation of post-change activation strength with search costs would rather indicate an unspecific role of frontopolar cortex in visual search than a specific role for change detection.
Subjects
Fifteen healthy volunteer students participated in the training session. One subject was excluded from the analysis because he was left handed. Another subject could not be used for RT-based analyses because her response times were not recorded in the scanner session, due to a coding error. The remaining 13 participants [12 female, 20–27 (mean 22.4) years old] had normal or corrected-to-normal vision and were naïve about the purpose of the experiment. Participants provided informed consent and were either paid or compensated with course credits. The experiment was approved by the local ethics committee.
Stimuli
Stimuli and procedures followed closely Experiment 1 by Chun and Jiang (1998)
. Each display contained one target (90° or 270° rotated T”) and 11 distractors (0°, 90°, 180°, 270° rotated L”). The orientation of the target was randomly chosen in each trial, so that its stem pointed either to the right or to the left. The color of both target and distractors was randomly selected among yellow, red, blue and green with the restriction that the four colors were equally frequent within the display. The background was always gray (RGB = 128, 128, 128). Each item subtended 0.64° × 0.64° and displays were generated by randomly placing items on an imaginary 8 × 6 grid that subtended approximately 12° × 4.9° of visual angle. The center position of each item was randomly jittered in steps of 0.16° (within a range of ±0.48° in visual angle along the vertical and horizontal axes) in order to prevent collinearities with other stimuli. See Figure 1
for schematic displays.
Figure 1. Sample displays. ‘New’ and ‘old’ refers to randomly generated and repeated distractor configurations, respectively. At the right, an ‘old’ display with new target location is shown. Displays are schematic, please refer to methods section for exact description.
Procedure
Training session
Stimuli were presented on a 19.7-inches color monitor (resolution of 1024 × 768 pixels, refresh rate of 60 Hz). Viewing distance was 70 cm, secured by a chinrest. The software ‘Presentation’, V. 10.03 was used to generate stimuli, to control the timing of the experimental events, and to record subjects’ responses.
Each experimental trial began with the presentation of a small white square centered on a gray background. Subjects were instructed to fixate the square. After 1000 ms, the fixation cross was replaced by the search display that remained visible until the participant made a target identification judgment by pressing either the left or the right button of the mouse, in accordance with the pointing direction of the stem of the T”. They were told to respond as fast as possible, without sacrificing accuracy. Subjects received a feedback about the correctness of their choice by means of two different sounds. A 10-s break was imposed after each block.
The experiment was framed into 30 blocks of 24 trials, for a total of 720 trials. For each subject, 24 target locations were randomly chosen at the beginning of the experiment. Twelve of them, balanced between the left and right halves of the screen, were assigned to 12 randomly generated configurations that were preserved during the experiment (“old” configurations), while the other 12 target locations, equally balanced between the two halves of the screen, were presented in each block with newly generated distractor configurations (new configurations”). In this way, frequency of target presentation was the same for all old and new configurations, ruling out target frequency differences to confound contextual cueing. The sequence of old and new trials was individually randomized for each participant. The target color and the colors of repeated distractors were kept constant. As described above, however, the orientation of the target ‘T’ was randomly varied, to prevent learning an association between a display configuration and a response.
fMRI session
On the day following the training session, participants took part in the fMRI session. The fMRI experiment was identical to the training experiment, with the following exceptions. Trial duration was 6 s. We (Pollmann et al., 1998
) and others (Huettel and McCarthy, 2000
) have previously shown that an interval of 4–6 s yields optimal power to detect blood oxygenation level dependent signal changes. After presenting the fixation cross for 2 s, search displays were presented until a response was given or a maximum of 4 s was reached. The session consisted of two epochs (i.e., 10 blocks). In each block, the 12 old configurations and the 12 new configurations were presented, randomly sequenced and intermingled with two null events (in which the fixation cross was presented for the whole length of the trial). The first epoch contained the same old displays as in the training session. In the second epoch, the set of target locations for the old configurations was swapped with the set of target locations for the new displays. Consequently, the old distractor configurations were not anymore predictive for the old target locations (i.e., the target locations used in the training session and the first epoch of the fMRI session). Exchanging the sets of target locations for old and new configurations ensured that presentation frequency for each location remained the same.
Stimuli were displayed by an LCD projector on a back-projection screen mounted in the bore of the magnet above the observer’s chest. Participants viewed the screen by means of a mirror positioned on top of the head coil.
Explicit recognition test
At the end of the fMRI experiment, participants conducted an explicit recognition test without previous notice. For this test, the 12 old configurations of the learning part of the experiment (training and fMRI-epoch 1) were sequenced randomly with 12 novel (i.e., not previously shown) displays, and subjects were asked for each display to indicate by an alternative forced choice button press whether or not they had seen the display in the experiment. The explicit recognition test was carried out after the end of the scanning, while participants were still lying in the scanner.
fMRI-Methods
FMRI data were acquired at 1.5T (GE Signa LX, General Electric, Milwaukee, WI, USA) with a GR-EPI sequence with TR = 2000 ms, TE = 40 ms and flip angle α = 80°. The fMRI-experiment consisted of 790 volumes of 23 horizontal slices acquired in ascending, interleaved order with 5 mm slice thickness and 1 mm gap. In-plane voxel size was 3.15 mm × 3.15mm. Data were acquired in a single run. The first 10 volumes were not analyzed to remove saturation effects. A conventional 2D spin echo sequence (TR = 520 ms, TE = 9 ms, matrix 256) was used to acquire structural images in the same plane as the functional images, which were used for registration with individual high-resolution anatomical 3D datasets.
FMRI data were analyzed using BrainVoyager QX (Brain Innovation, Maastricht, The Netherlands). The anatomical volume was transformed to the Talairach coordinate system (Talairach and Tournoux, 1988
) in three subsequent steps: first, voxel intensity was corrected for inhomogeneity (Vaughan et al., 2001
) and data were resampled to 1 mm resolution using a cubic spline interpolation. Second, data were aligned with AC-PC by means of cubic spline transformation and normalized into Talairach standard space using trilinear interpolation. Finally an automatic segmentation (Kriegeskorte and Goebel, 2001
) was performed to identify the boundary between gray and white matter, in order to perform a cortex-based data analysis.
For each subject, preprocessing of functional images included slice scan time and head movement correction using sinc interpolation and rigid body transformations with the first volume as the reference volume; linear trend removal, high pass filtering (frequency-space filter by FFT with frequency cutoff 0.02 Hz) and spatial smoothing with a 6-mm FWHM Gaussian filter. Functional images were then coregistered to the structural volume and transformed into Talairach space with a resolution of 3 mm × 3 mm × 3 mm using sinc interpolation.
Data Analysis
We carried out parallel multi-subject random effects general linear model (GLM) analyses on reaction time data and fMRI data. Error trials were removed from these analyses. For the fMRI analysis, event-related predictor functions were constructed using a classical two-gamma hemodynamic response function. Statistical maps were thresholded at a voxel-level threshold of p = 0.005 and a cluster-level threshold of p = 0.05 (Forman et al., 1995
). For the behavioral data, the significance criterion was α = 0.05. GLM analyses were followed by t-tests as described in results.
Relation of learning and change costs
We further wanted to know whether the amount of facilitation observed in the first, pre-change, epoch was related to the prolongation of search when the target changed in old displays. For this purpose, we first calculated a normalized prolongation score by subtracting the response times in the new trials of the first four post-change blocks from the old trials in the same blocks and dividing the difference by the mean of the new trials. The score was calculated over only four (instead of all five) post-change blocks because some relearning may have occurred in the fifth block, at least in a subset of participants (Figure 4
C). We then correlated the prolongation score with the facilitation score of the first epoch.
High and low facilitation subgroups
Due to the technical failure of response time recording in one subject, we could analyze the search times in 13 subjects. Out of these, we created equally sized subgroups of six participants each with the highest respectively lowest normalized response time differences between old and new displays of the first epoch and analyzed them in the same way as for the whole group.
Correlation with search duration
Signal increases after target location change may potentially reflect the longer search processes which were observed after the change in target location, instead of the hypothesized change detection. To investigate this issue, we correlated the individual beta value-increase for the old trials from block 5 to block 6 (the last block before and the first block after the change) divided by the beta-values of block 5 with the individual ‘old’ response time increase in the same blocks calculated equivalently.
Behavior
Training
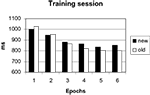
Errors occurred in only 1.2% of trials and were not further analyzed. The 30 blocks of the training session were grouped into 6 epochs of 5 blocks to increase statistical power. Search times were analyzed with a repeated measures ANOVA with configuration (old, new) and epoch (1–6) as factors. The main effect of epoch was significant [F(5, 70) = 84.99, p < 0.001], due to a general learning effect visible in both conditions (Figure 2
). The main effect of configuration was not significant [F(1, 14) = 0.65, p = 0.434], yielding no hint at an overall difference between configurations. Importantly, the interaction of configuration by epoch was significant [F(5, 70) = 3.57; p < 0.05], reflecting an added facilitation of search in the old displays which developed over epochs. Thus, the response times replicated the typical contextual cueing pattern.
Figure 2. Search times in the training session.
fMRI-experiment
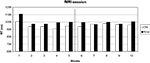
Only 0.5% errors were committed, probably due to the training on the day before. Errors were thus not further analyzed. An ANOVA on response times with configuration (old, new), change (before, after) and block (1–5) yielded significant main effects of configuration [F(1, 12) = 11.94, p < 0.05] and block [F(4, 48) = 2.80, p < 0.05] and a significant interaction of change by block [F(4, 48) = 8.03, p < 0.05]. All other main effects and interactions were not significant (all p > 0.53).
Figure 3
shows that the search times in old displays were faster than those in new displays before the target location change. After the change, this advantage was lost.
Figure 3. Search times in the fMRI session. Target location change occurred from block 6 on.
These differences were confirmed by separate ANOVAs with configuration (old, new) and block (1–5) on the first and second epoch. Epochs again consisted of five blocks, so that the pre-change and the post-change blocks formed one epoch each. The analysis of the first epoch yielded a significant effect of configuration [F(1, 12) = 4.93, p < 0.05] which disappeared in the second epoch [F(1, 12) = 0.677, p = 0.427].
Individual differences
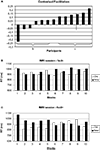
We had expected a selective increase of response times to accompany target location changes in displays with repeated distractor configurations. Whereas we found that the advantage for the repeated displays disappeared after the change in target location, the configuration by change interaction was nevertheless not significant. This prompted us to look for individual differences in the facilitation of search due to contextual cueing. Figure 4
A shows the normalized individual differences in search times in old and new displays in the first epoch of the fMRI experiment (i.e., before the target location change). Normalization was obtained by dividing the difference of ‘new’ – ‘old’ by ‘new’ search times. We observed a continuum from strong facilitation of search in old displays to the reverse, longer search times in old displays. The individual contextual cueing effects in our study were not random. The correlation of the facilitation by contextual cueing observed in the last epoch of the training and the first epoch of the fMRI experiment was r = 0.65 (p < 0.05).
Figure 4. Individual differences in the contextual cueing effect. (A) Normalized individual facilitation scores (see text for details). Search times for the high (B) and low (C) facilitation group.
We further wanted to know whether the amount of facilitation observed in the first, pre-change, epoch was related to the prolongation of search when the target changed in old displays. There was a high correlation between the two scores (r = 0.725; p < 0.05) indicating that the post-change increase in search times was highest in participants with strong facilitation by contextual cues.
Subgroup analyses
For the subgroup with high facilitation by contextual cues (Figure 4
B), the ANOVA with configuration (old, new), change (before, after) and block (1–5) yielded a significant main effect of configuration [F(1, 5) = 4.37; p < 0.05]. Importantly, the interaction of configuration by change was significant [F(1, 5) = 7.66; p < 0.05], as was the interaction of change by block [F(4, 20) = 3.32; p < 0.05]. All other main effects and interactions were not significant (all p > 0.244).
Following the overall ANOVA, we again analyzed the pre- and post-change epochs separately. In the first, pre-change, epoch, search times for old displays were significantly faster than for new displays [F(1, 5) = 99.36; p < 0.05], reflecting the selection criterion. There was also a significant effect of block [F(4, 20) = 3.45; p < 0.05], whereas the interaction was not significant [F(1, 5) = 0.75; p = 0.572]. In the post-change epoch, neither main effects nor the interaction were significant (all p > 0.140).
For the subgroup with low facilitation in the pre-change epoch (Figure 4
C) we observed a marginally significant main effect of configuration [F(1, 5) = 6.41; p = 0.052] and a significant change by block interaction [F(4, 20) = 3.95; p < 0.05], all other main effects and interactions (notably including the configuration by change interaction) were not significant (all p > 0.180).
In the ANOVA on the first epoch, only a significant main effect of block was observed [F(4, 20) = 4.13; p < 0.05; all other p > 0.543]. In the analysis of the second epoch, only a marginally significant effect of configuration [F(1, 5) = 6.00; p = 0.058] was observed. This reflected numerically higher search times in the new, rather than the old, configuration.
Thus, the subgroup which showed significant pre-change facilitation by contextual cues did also show a selective significant increase in search times in old displays following the target location change. This selective increase was not observed in the subgroup without significant pre-change contextual cueing.
Recognition
Finally, we analyzed the explicit recognition scores obtained at the end of the fMRI session. ‘Old’ responses were equally often hits (rate = 0.5055) and false alarms [0.5057; t(13) = 0.006, p = 0.995]. Analysis of high and low facilitation subgroups yielded no differences [high: hits/false alarms: 0.5467/0.5357; t(6) = 0.159, p = 0.879; low: hits/false alarms: 0.4583/0.4450; t(6) = 0.275, p = 0.794].
Imaging
In the same way as for the response time data, we analyzed the imaging data with a repeated measures ANOVA with configuration (old, new), change (before, after) and block (1–5).
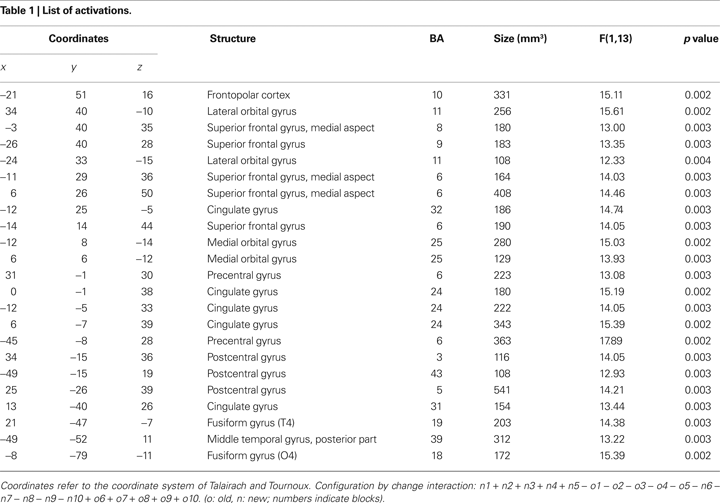
For the topic of this paper, selective signal changes after the change in target location in repeated distractor configurations are of central importance. We will therefore focus the analysis on the interaction of configuration by change. Although our main hypothesis concerned anterior prefrontal cortex, specifically left lateral frontopolar cortex, we refrained from using a region-of-interest approach in order to analyze selective target change-related responses across the whole brain. All activations are listed in Table 1
.
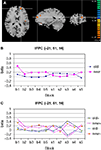
A significant interaction of configuration by change was observed in left lateral frontopolar cortex (BA10), as predicted (Figure 5
). Another area showing this activation pattern was observed somewhat more dorsally and posteriorly in left superior frontal gyrus (BA9). BOLD time courses measured in this area in the immediate pre- and post-change blocks are shown in Figure 6
. Significant configuration by change interactions were further observed in right lateral orbital gyrus, bilaterally in the posterior medial orbital gyri, at several frontomedian locations, in pre- and postcentral gyri, left posterior middle temporal gyrus and in the fusiform gyri (Table 1
).
Figure 5. Significant configuration by change interaction in left frontopolar cortex. (A) Location of left frontopolar activation. The frontopolar activation is marked by the cross hair. Somewhat more dorsally and posteriorly, the superior frontal gyrus activation is visible. Left hemisphere is presented on the right. (B) Group signal strength as a function of condition and block. The graph shows beta values, signifying the amplitude of the modeled BOLD-response. Old: repeated trials, new: novel trials. (C) Signal strength as a function of condition and block, separately for the high (+) and low (−) facilitation subgroups.
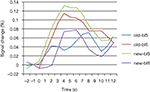
Figure 6. BOLD curves for frontopolar cortex as a function of condition and block. Curves represent the average event-related signal for old and new trials after subtraction of the null-event curves. Bl5/6: block 5/6.
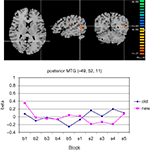
Among the posterior brain areas which showed a significant configuration by change interaction, the posterior segment of left middle temporal gyrus, in the TPJ area (Figure 7
) is of special interest, because this area may play a role in disengaging of attention from the learned target location to facilitate search for the new location.
Figure 7. Significant configuration by change interaction in left temporo-parietal junction area. (A) The TPJ-activation is marked by the cross hair. Left hemisphere is presented on the right. (B) Group signal strength as a function of condition and block. The graph shows beta values, signifying the amplitude of the modeled BOLD-response. Old: repeated trials, new: novel trials.
To find out whether the cross-over pattern observed in the ANOVA was due to negative signal changes, we carried out separate contrasts of pre-change old and post-change new displays versus null events. We found one area in left fusiform gyrus (−38, −50, −7) where new displays yielded lower signal amplitudes than null-events. There was no area where lower signal amplitudes were observed for pre-change old displays versus null events.
Correlation with search duration
We did not observe a significant correlation of activation strength and search time increase from block 5 to block 6 in any of the activated areas (see Section ‘Materials and Methods’ for details). Even without correcting for multiple comparisons, which is a conservative approach here, because it facilitates observation of a significant activation occurring by chance, no correlation was observed. There was also no significant correlation between signal change and RT-change from block 5 to block 7, calculated analogously. Thus, the post-change signal increase was not simply due to prolonged search.
High versus low facilitation
If left frontopolar cortex, as hypothesized, was involved in the detection of contextual changes or the interruption of attentional guidance to the old target location, it should have shown a more selective signal change in those participants who efficiently learned the target–context relation. Comparing the subgroups with high versus low search facilitation by contextual cues (Figure 4
A), we indeed observed a selective signal increase after target location change in old displays in the high facilitation group [F(1,5) = 13.58, p < 0.05] whereas no such effect was observed in the low facilitation group [F(1,5) = 0.003, p = 0.959; Figure 5
C]. Please not that both groups showed virtually identical activation in the pre-change epoch, with numerically higher activation for new trials. Only after the change did both groups show virtually mirror-image activation patterns.
Baseline differences
Many of the areas with a significant configuration by change interaction had, in absolute values, lower activation for repeated than novel displays before the target location change. We ran a conjunction analysis (Nichols et al., 2005
) between the configuration by change contrast and a new-old contrast for the first epoch. The conjunction was significant in the medial aspect of the left superior frontal gyrus, the left medial orbital gyrus and the left cingulate gyrus.
Our aim was to investigate the contribution of anterior prefrontal cortex to the implicit ‘detection’ of changes in implicitly learned stimulus contingencies in the service of optimal attention allocation. We used the contextual cueing paradigm to induce implicit learning of the spatial relation between target locations and distractor configurations. We then changed the target location, so that the learned distractor configuration lost its predictiveness as to the target location. When these changes occurred, subjects needed to change their gaze pattern to find the target. In a previous study we could show that subjects learn to redirect their gaze from a path leading to the learned target location to a new path which leads more efficiently to the new location (Manginelli and Pollmann, 2009
). Thus, subjects learn to reorient their overt attention (gaze) in a situation in which they are not aware that they had learned to efficiently use the distractor configuration to find the target in the first place and in which they are also not aware that this contingency between learned distractor configuration and target location changed.
Our hypothesis was that anterior prefrontal cortex is involved in detecting this change, although our subjects were not aware of it, as a precondition for subsequent attentional control processes necessary to adapt to the new situation. In support of this hypothesis, we observed a configuration by change interaction with an increase of activation for repeated displays after the change in target location in left lateral frontopolar cortex as well as left anterior superior frontal gyrus.
In addition to these anterior brain areas, selective post-change signal increases were also observed in a number of posterior brain areas. Some of these, like multiple activations along cingulate cortex and superior temporal activations, are in good agreement with the results of a recent tract-tracing study, which showed fibers from BA10 to terminate along the cingulate gyrus and in superior temporal cortex (Petrides and Pandya, 2007
). Of interest is also the absence of posterior parietal change-related activations, which concurs with the absence of rostral prefrontal fibers terminating in this area in the Petrides and Pandya study.
Among the areas which showed a selective post-change increase for old displays were further the posterior medial orbital gyrus (which is discussed below) and the posterior segment of the middle temporal gyrus in the temporo-parietal junction area, which we hypothesized to be a potential recipient of signals from frontopolar cortex, because of its role in disengaging attention from a current focus to facilitate a reorienting to new aspects of the environment (also discussed in more detail below). There is no evidence for monosynaptic connections between BA10 and TPJ (Petrides and Pandya, 2007
), so a communication between these areas will most likely be relayed over intermediary neurons.
Change of Implicitly Learned Contingencies
Search in displays with repeated (‘old’) distractor configurations was faster than in randomly generated, new configurations, replicating previous reports of contextual cueing. This search advantage was still visible 1 day after training, when the same old displays were presented in the scanner. However, when the target location was changed in old displays after learning, the search advantage was lost (in replication of Manginelli and Pollmann, 2009
). These learning effects distinguish the current data from a previous study, in which distractor configurations were repeated only six times before the target location change occurred, which was not sufficient to induce a significant facilitation of search times in repeated displays (Pollmann and Manginelli, 2009
). While anterior prefrontal change-related activation was observed in both studies, we could therefore not be certain to relate these changes to implicit learning of repeated contexts in the previous study. In contrast, in the present experiment, the size of the change-related activation in anterior prefrontal cortex scaled with the size of the facilitation due to context learning. In fact, only those subjects who showed a significant facilitation before the target location change also showed a significant increase of left frontopolar activation after the change.
While frontopolar activation depended on the strength of implicit learning, it was not correlated with the response time cost induced by the target location change in old displays. Thus, frontopolar cortex appears to support a specific process, the detection of change, rather than unspecific visual search processes.
We had observed that frontopolar activation was often observed in ill-structured situations, in the absence of explicit rules how to respond to the change (Pollmann, 2004
). The current experiment was designed to test the further hypothesis that frontopolar cortex is involved in change ‘detection’ (in an implicit sense) not only in the absence of rules or instructions to do so, but even if subjects are unaware of the change. We could show that our subjects could not distinguish between previously encountered and novel displays better than chance, confirming the hypothesis that even in the absence of change awareness, anterior prefrontal cortex is engaged.
This distinguishes our findings from previous findings of prefrontal responses to breaches of expectation. In one study, raising the proportion of invalid trials in a visuospatial cueing task elicited activation in orbitofrontal cortex, in the neighborhood of, but inferior to the frontopolar activation observed in the current study (Nobre et al., 1999
). In another study, violation of a temporal sequence led to more posterior prefrontal activation (Huettel et al., 2002
). Given that our subjects did not notice the change of target–distractor contingencies (nor the presence of such contingencies), the change-related signal changes cannot be explained by breaches of expectation. Thus, the discrepancies between the prefrontal areas activated in the present study and those activated by breaches of expectation are not unexpected. Another noteworthy aspect is that spatial contextual cueing and temporal sequence learning, although structurally similar, appear to rely on different anatomical circuits (e.g., Negash et al., 2007a
,b
).
Contextual Interference
In the contextual change paradigm applied here, anterior prefrontal cortex appears to subserve an implicit control function. It comes into play when implicitly learned context relations are no longer predictive of the target location and attention (including overt eye movements) needs to be directed elsewhere in search of the target. As outlined in the introduction, studies of memory have implicated lateral frontopolar cortex in episodic retrieval processes. Episodic retrieval would appear to be a prerequisite for the detection of task-relevant change. The memory literature suggests that frontopolar cortex becomes active when previously attended or memorized objects reappear in a changed context (King et al., 2005
). This was also the case in the present study, where displays with repeated distractor configurations contained a target at a new location. Critically, the present study shows that this context retrieval does not demand previous explicit encoding.
Repetition Suppression
Repetition of a stimulus can lead to adaptation of neural responses as well as the BOLD response (e.g., Grill-Spector et al., 2006
). It is unlikely, however, that repetition suppression is the neural process which leads to the facilitation of visual search due to contextual cueing. Repetition of the distractor configuration could lead to a ‘fading’ representation of the display which, in turn, makes the target more salient, leading to faster search times. In this case, however, a target presented at a new location in a repeated display should be as salient, or even more salient than, a target at the old location (Manginelli and Pollmann, 2009
). Repetition suppression thus cannot explain the loss of facilitation in old displays following the target location change. This does not rule out that neural responses to repeated displays may be reduced in some brain areas, particularly in visual cortex. However, when we investigated this question recently, we observed no change of signal amplitude following repeated displays, but rather an earlier onset of the BOLD response compared with novel displays (Pollmann and Manginelli, accepted
).
Interference in New Displays
An unexpected finding was the decrease of activation for new displays after the change in target location, which was consistently observed across anterior and posterior brain regions (Figures 5 and 7
). This pattern almost looks as if there was a limited capacity that needs to be distributed between old and new trials, so that increased demand for old displays, for example, after the target location change goes along with a redistribution of resources from the new to the old trials. However, it remains currently unclear how such a redistribution should proceed in the absence of voluntary resource allocation.
Individual Differences
In keeping with a previous report (Lleras and von Mühlenen, 2004
), we observed a considerable range of interindividual degrees of search facilitation. Importantly, the activation patterns reflected these differences in that post-change signal increase in all investigated areas (for reasons of space only shown for frontopolar cortex, Figure 5
C) was higher in participants with efficient usage of contextual cues, mirrored by strong initial search facilitation and subsequent pronounced post-change search costs.
The individual differences replicate closely a previous report of a very similar continuum of individual responses to contextual cues (Lleras and von Mühlenen, 2004
). As in the study of Lleras and von Mühlenen, the individual effects in our study were not random. There was a high correlation of the facilitation by contextual cueing observed in the last epoch of the training and the first epoch of the fMRI experiment, on the next day. Interestingly, a subset of three participants showed (at least in absolute terms) slower responses for repeated than for new trials. As mentioned above, contextual cueing relies on both facilitatory and inhibitory processes (Ogawa et al., 2007
). It may be worthwhile to investigate whether in some individuals, the target location may be inhibited along with the distractor locations in repeated displays. This could explain the slower response to repeated displays.
Relation of Frontopolar and Posterior-Temporal Change-Related Processes
The temporo-parietal junction area, which has been proposed to support changes of attention away from a current focus to new aspects of the environment (Corbetta et al., 2008
) showed a configuration by change interaction. It makes sense that TPJ would be more active after the change in target location in old displays, because attending to the old, implicitly learned target location needs to give way to learning the new location. Typically, TPJ activation has been observed in response to salient events in the environment (e.g., Friedrich et al., 1998
; Corbetta and Shulman, 2002
). In the absence of such salient events, as in the present study, in which the change of target–distractor context was not even consciously recognized, anterior prefrontal cortex may play a vital role in signaling task-relevant changes to posterior brain areas, such as the TPJ, as a precondition for adaptive reallocation of attentional resources to changed aspects of the environment. Clearly, this relationship awaits further tests in which a higher number of old displays allow the investigation of the immediate post-change processing with higher statistical power.
The cortex along the middle part of the intraparietal sulcus may also contribute to the attentional selection of competing stimuli (Molenberghs et al., 2008
). We found no evidence for a differential involvement of this structure in the conditions of the present experiment. This may be due to the fact that this area is also strongly involved in reorienting of attention (Vandenberghe et al., 2001
) and was therefore comparably active in old as well as new trials, before and after the change, given that visual search in this study was inefficient, demanding multiple saccades in a typical trial.
Differentiation of Past and Present
Our proposition that frontopolar cortex is involved in implicit contextual change detection rests on the idea that old relations between an object and its context, which have been helpful to search for the object in the past, need to be replaced by new object–context relations if these relations have changed in the environment. In other words, previously learned contextual relations need to be tagged as memories of the past, whereas the new contextual relations need to be tagged as presently relevant. This same distinction between memories of the past and memories which are relevant for guiding present behavior breaks down in patients with spontaneous confabulations (Schnider, 2003
). These patients commonly have lesions of the basal forebrain and medial orbitofrontal cortex. Moreover, activation of the basal forebrain increased with increasing repetition of stimuli in a memory task when only the last repetition was task-relevant and previous occurrences needed to be suppressed (Schnider et al., 2000
). In the present study, selective post-change signal increases for repeated displays in basal forebrain and orbito-frontal cortex may well indicate suppression of the old target context relations in favor of the new.
Laterality
Change-related activation in anterior prefrontal cortex was lateralized to the left hemisphere. This replicates previous findings of dimension- and location-change-related activation changes (Pollmann et al., 2000
; Weidner et al., 2002
) or anterior prefrontal lesions (Pollmann et al., 2007
). Reviews of episodic retrieval studies indicate a preponderance of right frontopolar activations, along with a sizeable number of bilateral frontopolar activations, but a scarcity of left-frontopolar only activations (Christoff and Gabrieli, 2000
). In contrast, Reynolds et al. (2006
) report a left lateral frontopolar involvement in integrating contents retrieved from episodic memory. Taken together, this may suggest that the left lateral frontopolar cortex does not subserve pure retrieval of old contextual information from memory, but processes this information, perhaps trying to integrate the current context with similar previously learned contexts and signaling if this is not possible.
One crucial question which remains open is whether left lateral frontopolar cortex is involved only in detecting changes between past and present episodes or whether it generates control signals that instruct more posterior areas, e.g., in the temporo-parietal junction area, to start a process cascade of attentional disengagement from the old and engagement to a new aspect (such as a new location or feature dimension) of the environment.
Scope of Explanation
Since anterior prefrontal function has become a focus of research in recent years, lateral frontopolar activation has been reported in many different paradigms. It remains an open question whether these findings are due to a common process or whether different paradigms tap different functions which are subserved by frontopolar neurons. In other words, can the implicit detection of attention-relevant environmental changes explain anterior prefrontal activations in other paradigms? Clearly, this remains speculative at the moment and awaits further tests, including direct comparisons between implicit attention change, memory retrieval and explicit change processes. Some candidate processes are discussed below.
It has been proposed that lateral frontopolar cortex is selectively involved in branching (Koechlin et al., 1999
). Branching is characterized by holding information about an interrupted task in memory while carrying out a second task. For branching, it is crucial, that enough information about the task contents of the primary task is held in memory to be able to continue it when the second task is finished, as compared to a dual task situation, in which one of two tasks is alternately worked on. It appears that branching is thus characterized by a stronger ‘contextual interference’ component, in that current stimuli need to be manipulated according to the rules of one task when the rules (and stimuli) of the previous task are still held available. The same holds in general for situations of subgoal processing, which also lead to lateral frontopolar activation (e.g., Braver and Bongiolatti, 2002
). Similarly, relational integration (Christoff et al., 2001
) is characterized by two contexts, which first need to be processed separately and then being integrated to solve the task. A recent classification analysis yielded a distinction between episodic memory demands in the most lateral and multitasking in the more anteromedial parts of lateral frontopolar cortex (Gilbert et al., 2006
). Integration of two episodic memory contents has been postulated to be a function specifically of left lateral frontopolar cortex (Reynolds et al., 2006
). Recently, lateral frontopolar cortex has been reported to respond differentially to the validity of working memory contents used in visual search (Soto et al., 2007
). The authors’ view that anterior prefrontal cortex may compare internal and external representations and prioritize them in accordance with task goals also fits with our concept of left lateral prefrontal function.
The proposed role of lateral frontopolar cortex in detecting contextual change and potentially suppressing the old context may thus specify one aspect of frontopolar function that falls into the broad concepts of evaluating self-generated information (Christoff and Gabrieli, 2000
), a ‘gate-keeper’ function between stimulus-oriented and stimulus-independent processes (Burgess et al., 2005
), or the integration of several mental processes (Christoff et al., 2001
; Ramnani and Owen, 2004
). But again, the decisive difference is that these concepts were developed with explicit executive functions in mind, whereas we show that frontopolar cortex is involved in changes of implicitly learned configurations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Deutsche Forschungsgemeinschaft, grant PO 548/6-2.