1
Olin Neuropsychiatry Research Center, Institute of Living, Hartford, CT, USA
2
Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA
3
Department of Electrical Engineering, University of New Mexico, Albuquerque, NM, USA
4
The Mind Research Network, Albuquerque, NM, USA
In complex genetic disorders such as schizophrenia, endophenotypes have potential utility both in identifying risk genes and in illuminating pathophysiology. This is due to their presumed status as closer in the etiopathological pathway to the causative genes than is the currently defining clinical phenomenology of the illness and thus their simpler genetic architecture than that of the full syndrome. There, many genes conferring slight individual risk are additive or epistatic (interactive) with regard to cumulative schizophrenia risk. In addition the use of endophenotypes has encouraged a conceptual shift away from the exclusive study of categorical diagnoses in manifestly ill patients, towards the study of quantitative traits in patients, unaffected relatives and healthy controls. A more recently employed strategy is thus to study unaffected first-degree relatives of schizophrenia patients, who share some of the genetic diathesis without illness-related confounds that may themselves impact fMRI task performance. Consistent with the multiple biological abnormalities associated with the disorder, many candidate endophenotypes have been advanced for schizophrenia, including measures derived from structural brain imaging, EEG, sensorimotor integration, eye movements and cognitive performance (Allen et al., 2009
), but recent data derived from quantitative functional brain imaging measures present additional attractive putative endophenotypes. We will review two major, conceptually different approaches that use fMRI in this context. One, the dominant paradigm, employs defined cognitive tasks on which schizophrenia patients perform poorly as “cognitive stress tests”. The second uses very simple probes or “task-free” approaches where performance in patients and controls is equal. We explore the potential advantages and disadvantages of each method, the associated data analytic approaches and recent studies exploring their interface with the genetic risk architecture of schizophrenia.
Why are Endophenotypes Important? Definitions of Endophenotypes versus Biomarkers and Major Implications
Schizophrenia is an inherited, complex genetic disorder currently defined categorically on the basis of cross-sectional symptoms and longitudinal course, but not underpinned by any objective biological measures or physical/neurologic signs. This is problematic as:
(a) schizophrenia is complex at the level of the phenotype, due to high variability and mutability of the defining clinical symptoms.
(b) patients may refuse to disclose their symptoms (due for example to paranoid suspiciousness, itself a common symptom of the illness), or
(c) individuals may claim falsely to have symptoms (for example in a legal context), that are by nature unverifiable and
(d) although the clinical symptomatology of schizophrenia can be striking, there are no pathognomonic symptoms, (for example considerable overlap exists between symptoms of schizophrenia and those of psychotic bipolar disorder).
Because the pathophysiology of schizophrenia is obscure, there is no laboratory test or biological marker deriving from the core etiopathology. Biomarkers are quantitative characteristics that signal normal or abnormal biologic processes, or predict treatment response. For particular pathologic states they are disease-specific flags of its existence or severity, directly associated with clinical manifestations and outcome (Allen et al., 2009
; Ritsner and Gottesman, 2009
). For example, hemoglobin A1c (or glycosylated hemoglobin) in Type II diabetes is related both to pathophysiology (altered carbohydrate metabolism) and indicates an important disease feature, (abnormally elevated blood glucose). Because the classical phenotype of schizophrenia is complex, varied and overlaps extensively with that of other illnesses such as psychotic bipolar disorder, the search for biological markers associated with schizophrenia has been a difficult one.
Because of such problems investigators in recent years have focused more on endophenotypes then on biomarkers. In contrast to biological markers, endophenotypes or “intermediate phenotypes,” are viewed as quantifiable biological variations or deficits that are examples of stable trait markers or indicators of presumed inherited disease vulnerability (for recent reviews, see Prasad and Keshavan, 2008
; Allen et al., 2009
; Ritsner and Gottesman, 2009
). Endophenotypes, as conceived by Gottesman and colleagues (e.g. Gottesman and Gould, 2003
; Chan and Gottesman, 2008
) and elaborated by others (Pearlson and Folley, 2008a
,b
; Prasad and Keshavan, 2008
; Allen et al., 2009
), are heritable, quantitative traits associated with an illness both epidemiologically and also conceptually in the sense of being on the putative path from genes, via molecular biologic mechanisms, to brain states to overt behavior. They are state-independent (i.e. not only present during acute illness), co-segregate within families and occur in some unaffected relatives of individuals with the disorder, (because they represent vulnerability for the disorder), although at a higher prevalence than in the general population. They may not be visible to the naked eye and are generally assessed by experimental, laboratory-based methods rather than by clinical observation; this approach may include challenge tests to “unmask” the marker. Because schizophrenia is likely a common, multi-genetic disorder (analogous to hypertension or type II diabetes) endophenotype strategies are increasingly used by researchers, based on the presumption that endophenotypes are more straightforwardly inherited and are underpinned by fewer genes than are complex, heterogeneous phenomenological entities such as clinical psychiatric diagnostic categories (Pearlson and Folley, 2008a
,b
). Because endophenotypes are “intermediate” between a clinical syndrome and the associated disease vulnerability genes (illness markers not illness features) using them therefore simplifies the search for the etiopathology and genetic determinants of schizophrenia (Chan and Gottesman, 2008
; Pearlson and Folley, 2008a
,b
).
The reader is referred to a recent review of schizophrenia-associated endophenotypes (Allen et al., 2009
). Despite displaying some useful properties (Prasad and Keshavan, 2008
), employment of structural brain imaging endophenotypes in schizophrenia has generally been limited by their low diagnostic specificity. Endophenotypes derived from functional imaging paradigms seem intuitively more promising, but there is an enormous variety of these from which to choose. This article contrasts two very different such functional endophenotypes; those associated with working memory (WM) tasks versus those related to simple or no cognitive tasks. As well as task type, analytic strategies also vary. The majority of task-related functional studies in schizophrenia are analyzed using classic general linear model (GLM) based approaches; recently, newer analytic paradigms such as independent component analysis (ICA) have opened up new possibilities for both task-related and unrelated designs.
There are problems inherent in the predominant fMRI research strategy of focusing on challenge tasks based in cognitive domains where schizophrenia patients are behaviorally impaired, such as WM. Such problems include that patients often do not fully comprehend complex instructions and have problems performing tasks consistently in the scanner. They fatigue easily, have generally reduced concentration and attention, may be poorly motivated, distracted by illness symptoms such as hallucinations and sedated from medication side effects. Poor performance and abnormal task-related BOLD response are thus confounded in a “chicken and egg” situation which may be difficult to disambiguate. One solution to this problem has been to use easy or minimal-effort paradigms such as oddball tasks in which patients and controls perform at comparable levels of accuracy, or even imaging during rest, when there is no task (such as resting state/default mode paradigms), requiring no cognitive effort on the part of the subject (e.g. Greicius et al., 2004
; Bluhm et al., 2007
; Garrity et al., 2007
).
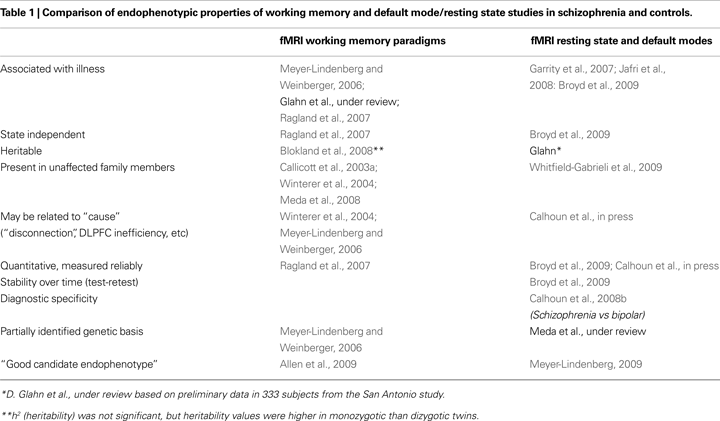
Some of the major contrasts between these two divergent types of studies discussed in this article are highlighted in Table 1
, which cites representative and recent widely cited illustrative articles and reviews whose general topic headings are elaborated in detail in the remainder of the paper.
Current Neuroimaging Approaches Using Cognition; DLPFC in Patients and in Relatives
Working memory, the ability to hold information on-line and manipulate it for short periods of time (Baddeley, 1992
) has been studied in depth in humans and animals. WM and related executive abilities, (e.g. planning, multi-tasking), are characteristically impaired in schizophrenia, Silver et al. (2003)
. Schizophrenia patients exhibit deficits on WM tasks of many designs (Park and Holzman, 1992
; Cohen et al., 1996
; Barch et al., 1998
, 2009
; Goldberg et al., 1998
; Wexler et al., 1998
; Park et al., 1999
). Such WM performance disturbances in schizophrenia are present in never-treated, first-episode, acutely ill and chronic patients and also (to a lesser degree) in their unaffected first-degree relatives, including discordant twins (Callicott et al., 2003a
; Barch and Smith, 2008
; Meda et al., 2008
). Issues related to WM abnormalities in schizophrenia have become methodological test-beds in the research field as the allied pathophysiology has been so well studied in this disorder and reflects daily life functioning (Green, 1996
).
For these reasons, different types of WM paradigms have been chosen as the basis of dominant type of cognitive functional MRI task studied in schizophrenia patients. Following on WM studies of nonhuman primates (Friedman and Goldman-Rakic, 1994
; Petrides, 1995
; Miller et al., 1996
), human WM fMRI studies have largely focused anatomically on the dorsolateral prefrontal cortex (DLPFC), a similarly involved area in humans (D’Esposito et al., 1999
; Rypma and D’Esposito, 1999
; Manoach et al., 2003
; Veltman et al., 2003
). DLPFC likely plays a crucial role in coordinating a distributed, executive task-relevant functional network. Additional modules in this circuit include other frontal regions, (ventrolateral and polar PFC and anterior cingulate), plus inferior parietal lobule (Manoach et al., 2003
; Meda et al., 2008
) and hippocampus (Glahn et al., 2005
; Meda et al., 2008
).
fMRI studies in schizophrenia typically center on patient/control DLPFC activation differences. Reports disagree on the direction of differences (see meta-analysis of Van Snellenberg et al., 2006
), with some findings of patient DLFPC underactivation compared to controls (Yurgelun-Todd et al., 1996
; Callicott et al., 1998
) and others of patient overactivation (Manoach et al., 2000
; Callicott et al., 2003b
). Evidence suggests that the magnitude and direction of BOLD response vary depending on relative task difficulty in relation to a given individual’s baseline efficiency on a particular task, (Manoach et al., 2000
; Callicott et al., 2003b
; Johnson et al., 2006
; Meda et al., 2008
). Thus, under conditions of equivalent task performance, schizophrenia patients activate DLPFC “inefficiently,” manifesting more WM-related activation than controls (Callicott et al., 1999
, 2003b
). With increasing task difficulty, patients exceed their cognitive capacity, leading to their disengaging or performing poorly, with consequent relative DLPFC underactivation (Manoach 2003
; Callicott et al., 2000
, 2003b
; Manoach et al., 2000
; Johnson et al., 2006
). Thus, WM load correlates with DLPFC activation in an inverted U-shaped curve; the curve in schizophrenia is both be flatter and shifted towards the left compared to controls, reflecting their inefficient task-related BOLD- response (Callicott et al., 2003b
; Johnson, et al., 2006
). Schizophrenia may also be associated with reduced ability to use context to guide task performance (Cohen et al., 1996
; Servan-Schreiber et al., 1996
; Barch et al., 2001
; Henik et al., 2002
; Ford et al., 2004
; Johnson et al., 2006
).
Different WM investigations in schizophrenia have utilized different task designs to best highlight particular aspects of abnormal responding in patients. Load vs BOLD response effects are most often demonstrated by measuring activation at several levels of increasing memory load, often by using N-back WM tasks (e.g. Callicott et al., 1999
, 2000
; Perlstein et al., 2001
; Jansma et al., 2004
). N-back designs however have unavoidable design problems. Usually, they incorporate target stimuli as probes, conflating the separate WM subprocesses of encoding, maintenance, and retrieval. Ideally, these are modeled separately, as their underlying functional anatomy may differ. Also in schizophrenia, the different subprocesses may be differentially impaired. Second, the steep difficulty gradient of the task curtails WM load-related response to three WM difficulty levels. Related to this, the 1-back level is generally easy for both control and schizophrenia subjects, but the 3-back condition exceeds WM capacity in many patients and some healthy controls. Patients, aware of their poorer task performance at more difficult levels, can become demoralized, unmotivated and disengaged from the task.
For the above reasons, some investigators have preferred to use versions of the Sternberg Item Recognition Paradigm (Sternberg, 1966
) to examine WM (Manoach et al., 1997
, 2000
, 2003
; Veltman et al., 2003
; Johnson et al., 2006
; Meda et al., 2008
) because WM load can be increased more gradually and the distinct task stages separated more easily; relative to the N-Back task, the Sternberg task allows a clearer temporal dissociation of encoding, maintenance, and response selection/response selection phases of WM.
Despite the numerous WM studies in the schizophrenia literature, and the demonstration of abnormal cortical connectivity (Meyer-Lindenberg et al., 2001
), several important questions remain to be clarified regarding the specific neural underpinning of impaired cognition in schizophrenia. For example, task-related DLPFC activation in schizophrenia is often more diffuse, less restricted to DLPFC and more likely to involve antero-medial and ventral frontal activation., This phenomenon is not related to the specific WM task employed, but could represent a result of DLPFC inefficiency, resulting in the need for backup recruitment of neighboring regions (e.g. as argued by Glahn et al., 2005
; Ragland et al., 2007
). In addition, the epicenter of DLPFC activation in schizophrenia differs from that identified in controls, being located in regions close by (Glahn et al., 2005
). This phenomenon could either be based on abnormal functional connectivity, or on deviant structurally-based functional localization e.g. see MacDonald et al. (2006
). While a more recent quantitative meta-analysis (Minzenberg et al., 2009
) discusses aspects of this issue, the more over arching question has to do with the fundamental underlying mechanisms. Until these are better clarified for example in the biochemical or genetic level the resulting phenomena (i.e. reduced motivation etc.) introduce design issues that cloud the ability to integrate fully across levels of measurement.
With regard to identifying the precise nature and progression of WM abnormalities in schizophrenia (and unaffected relatives), one debate centers on the specificity of WM deficits to task phase (e.g. MacDonald et al., 2003
). Future studies will likely focus more on modeling the maintenance period between encoding and retrieval, which has been relatively understudied, but may be crucial. For example, Driesen et al. (2008)
showed reduced prefrontal activity in patients during the maintenance phase, related to a faster decay rate of activity over time.
In summary, abnormal WM-related fMRI activation in occurs in a network, not just a single region (the DLPFC). The network in schizophrenia patients performs less efficiently, is less context-responsive to and does not react smoothly to changing load demands; the maintenance period may be especially impaired. Both hyper- and hypo-activation in patients are explainable by an inability to efficiently organize and distribute appropriate circuit resources as needed for effective WM performance.
Abnormal WM-based fMRI BOLD response was chosen as a potential schizophrenia endophenotype based on findings of WM performance deficits in non-affected siblings of schizophrenia patients and in discordant twin studies (Park et al., 1995
; Goldberg et al., 2003
; MacDonald et al., 2003
). As predicted, in fMRI studies, unaffected siblings also show aberrant DLPFC activation during WM tasks, even in some cases in the face of normal task performance, emphasizing the point made earlier that the endophenotype may be closer to the pathologic mechanism than to overt behavior. For example Callicott et al. (2003a)
found increased DLPFC activation in unaffected sibs of patients versus controls during encoding and manipulation of information, despite normal task performance. Brahmbhatt et al. (2006)
determined that high-risk siblings abnormally hyperactivated PFC during response selection. Thermenos et al. (2004)
, using a combined attention/WM task, showed unaffected relatives had more task-related activation in prefrontal cortex and thalamus; when task performance was controlled, relatives over-activated. Finally, Meda et al. (2008)
in an fMRI Sternberg WM task, reported that performance accuracy in unaffected first-degree relatives did not differ from controls (although relatives were slower in responding to probes). The major functional differences were that relatives hypo-activated bilateral dorsolateral/ventrolateral prefrontal cortices (DLPFC/VLPFC) and the posterior parietal cortex during stimulus encoding epochs and hypo-activated bilateral DLPFC and parietal areas during response selection. fMRI differences in both conditions were load-modulated, with a parametric increase in between-group differences with load in key regions during encoding and an opposite effect during response selection. While Callicott et al. (2003a) and Thermenos et al. (2004)
, reported increased DLPFC activation, Meda found DLPFC underactivation in both encoding and response selection task phases, likely related to differences in task design or difficulty, as discussed earlier. Thus in sum, (as reviewed by Meyer-Lindenberg and Weinberger, 2006
), abnormal WM fMRI responses in unaffected relatives in addition to those in patients confirmed their suitability as potential endophenotype candidates.
A richer explanatory context is now emerging for the abnormal fMRI WM findings in schizophrenia. As we mentioned above, an earlier, simpler approach was to view schizophrenia as a primary DLPFC defect, with resulting WM deficits underlying both other cognitive deficits and major positive symptoms of the disorder (e.g. see Cohen et al., 1996
; Silver et al., 2003
) However, cognitive neuroscience suggests that network-level abnormalities at the level of circuits can better account for the WM abnormalities in schizophrenia than explanations based on a single region, in a manner that also has interesting implications consistent with the concept of schizophrenia as a “disconnection syndrome” (Friston and Frith, 1995
). Evidence for this hypothesis is emerging from ICA, a data-driven approach especially useful for decomposing activation during complex cognitive tasks where multiple operations may occur simultaneously. It is often used to identify temporally coherent networks (Calhoun et al., 2008a
) as we discuss later.
Second, fMRI reveals that tasks other than WM can produce abnormal BOLD signal in DLPFC in schizophrenia (eg Winterer et al., 2004
; Becker et al., 2008
; Delawalla et al., 2008
; Woodward et al., 2009
). Some of these paradigms (e.g. choice reaction time; Woodward et al., 2009
), are based on tasks whose performance is heritable, associated with genetic vulnerability for schizophrenia in twin studies and state-independent in patients, suggesting they are also endophenotype candidates.
Third, regions other than DLPFC, and not necessarily strongly connected to it, also behave abnormally in schizophrenia patients and their siblings (e.g. Vink, 2006
; Bonner-Jackson et al., 2007
). The anterior cingulate cortex is but one example of a brain region forming part of a network that is severely disrupted in schizophrenia across many cognitive paradigms ranging from the complex (e.g. conflict monitoring/cognitive interference; Rubia et al., 2001
; Heckers et al., 2004
; Kerns et al., 2005
) to the simple, (e.g. auditory oddball detection (AOD) (Kiehl et al., 2005
; Laurens et al., 2005
). Additionally other types of cognitive tasks such as sentence completion also provoke abnormal network BOLD activation in unaffected siblings of schizophrenia patients (e.g. Whalley et al., 2005
).
These network-related abnormalities in general could represent an underlying, unifying issue of central importance to schizophrenia, for example an abnormality in underlying dopaminergic “tuning” or efficient signal transduction.
Finally, as would be expected of an endophenotype, a genetic context for WM fMRI studies in schizophrenia is now emerging; such studies to date have generally examined a single risk gene, (e.g. Egan et al., 2001
). Callicott et al. (2003b)
examined effects of COMT met/val genotype on PFC fMRI activation in several performance-matched diagnostic groups, during an N-back task. Irrespective of diagnosis (schizophrenia, unaffected sib, control), met allele load predicted more efficient physiological response (less PFC BOLD activation) in the 2-back condition. In other words, “the group with relatively more cortical dopamine available at the synapse (i.e. met homozygotes) had relatively greater behavioral “bang” for its physiological “buck”. In both cohorts, siblings and schizophrenia patients showed increased DLPFC activation (inefficiency) relative to controls, despite comparable performance, suggesting heritability of inefficient PFC activation. The fact that inefficient DLPFC activation is not straightforwardly correlated with abnormal WM performance in relatives and is associated with schizophrenia risk genes such as COMT polymorphisms, supports its utility as a presumptive endophenotype as also suggested by Goldberg et al. (2003)
.
There are parallels to the above, for example that allelic variation in putative schizophrenia risk gene SNPs also influence hippocampal activation during several cognitive fMRI tasks; as do a Ser704Cys SNP of the disrupted in schizophrenia-1 (DISC1) gene, and a common variant of the brain-derived neurotrophic factor gene, (Egan et al., 2003
; Pezawas et al., 2004
; Callicott et al., 2005
; Di Giorgio et al., 2008
).
To summarize the above, Karlsgodt et al. (2008)
summarized that evidence across such studies implicates neurodevelopmental disruption of brain connectivity in schizophrenia likely involving susceptibility genes affecting development of intra- and inter-regional connectivity. Similarly Tan et al. (2007)
suggests that a final common effect of dopaminergic (e.g. COMT) and glutamatergic (e.g. GRM3) risk genes on “macrocircuit stability and functional efficiency” affect cortical signaling and ultimately processing strategies, leading to the characteristic cognitive deficits in schizophrenia. Thus, patients engage larger networks of cortical regions during task performance, consistent with “reduced signal-to-noise components and the recruitment of compensatory networks”.
There are problems inherent in the predominant fMRI research strategy of focusing on challenge tasks based in cognitive domains where schizophrenia patients are behaviorally impaired, such as WM. As we discussed earlier, numerous illness-related factors confound abnormal task-related brain activation and poor task performance. One solution to this problem has been to use easy or minimal-effort paradigms such as oddball tasks in which patients and controls perform at comparable levels of accuracy, or even imaging during rest, when there is no task (such as resting state/default mode paradigms), requiring no cognitive effort on the part of the subject (e.g. Greicius et al., 2004
; Bluhm et al., 2007
; Garrity et al., 2007
).
Several groups have studied the AOD paradigm, originally developed in event-related potential studies, in detail using fMRI because it is a straightforward, relatively simple task activating multiple, diverse cortical and subcortical regions and is abnormal in schizophrenia patients and their unaffected relatives (Winterer et al., 2003a
,b
). Functional brain imaging studies took advantage of this existing task which could be extended informatively with the unique capabilities of functional MRI. Despite the fact that patients can perform the task almost as well (as accurately if slightly slower than) healthy controls, GLM-derived activation patterns are abnormal in most schizophrenia patients (Calhoun et al., 2004
; Kiehl et al., 2005
; Calhoun et al., 2006a
,b
,c
; Garrity et al., 2007
; Demirci et al., 2009
; Sui et al., 2009
). The electrophysiological equivalent of the auditory oddball fMRI task is the auditory oddball P300 paradigm, a recognized endophenotype candidate for schizophrenia, whose activation patterns are strongly heritable, minimally influenced by illness stage or antipsychotic medication, and often abnormal in first-degree unaffected relatives (although not necessarily diagnostically specific). Future auditory oddball studies will undoubtedly explore more endophenotypic properties of fMRI response in schizophrenia such as heritability estimates, specificity of abnormal patterns to schizophrenia, responses in unaffected first-degree relatives etc. In addition to primary task-correlated BOLD patterns generally obtained through use of GLM analytic approaches, (e.g. Kiehl et al., 2005
) the AOD when analyzed using Independent Component Analysis (ICA)-based approaches (e.g. Calhoun et al., 2004
, 2008b
), has been used as a convenient means to derive default mode data (see below).
Complex cognition is not generated by local processing within a single task-engaged brain region such as DLPFC, but from widely distributed groups of brain regions acting as neural networks or circuits (Ramnani et al., 2002
; Fuster, 2006
). In addition to the well-documented networks underlying WM, vision, language, sensory, motor and focused attention, researchers were surprised to discover other sets of networks (typically 10 or so) unrelated to overt cognitive tasks and even present at rest when no task was being performed (Cordes et al., 2001
), (so called “resting state networks” or “default mode”) in which the brain idles, but there is interconnected processing of activity among major centers in the cerebral cortex (Raichle et al., 2001
) discussed below. Examination of spontaneous brain activity during “rest” potentially eliminates the type of behavioral performance difference-related confounds mentioned at the start of this section and captures differences in “baseline” cognitive activity.
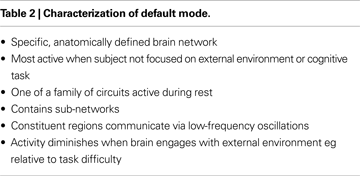
Characteristics of the Default Mode are listed in Table 2
.
A major statistical approach to identifying signal from such networks is ICA. ICA and similar techniques are methods for recovering underlying signals from linear signal mixtures using higher-order statistics to determine a set of components that are maximally independent of each other. The method is “blind,” so that no task-related time course information is required in the model. ICA also has the advantage of not requiring seed voxels or the use of temporal filtering, (see McKeown and Sejnowski, 1998
; Broyd et al., 2009
). ICA is based on the assumption of spatially independent, temporally correlated, coherent brain networks. In addition to the strong temporal correlations within each component/network, ICA approaches can also be used to identify also weak temporal correlations among these different networks. The latter relationships, as we review later, are used to assess functional network connectivity. ICA has been used to identify several temporally coherent networks present in healthy subjects either during rest or during performance of various tasks.
This type of functional connectivity is often measured as inter-regional correlations among spontaneous fluctuations of hemodynamic activity during a “resting state” while participants lie passively in the MRI machine, but no active cognitive or behavioral demands are imposed. Initial identification of significant temporal inter-correlation among the precuneus/posterior cingulate, ventral anterior cingulate, and ventromedial prefrontal cortex (i.e., regions now defined as comprising the classic “default mode” of brain activity) (Greicius et al., 2003
; Buckner et al., 2008
; Broyd et al., 2009
) led to interest in locating additional functionally-integrated neural networks during resting state and soon led to related fMRI research in schizophrenia. These recent reviews speculate that several mesial temporal regions may also contribute a sub-network within the default mode, but it remains unclear whether this contribution is primarily from memory-related functional studies. Investigations applying ICA or similar methods to resting state fMRI data have identified additional discrete neural circuits comprised of brain regions often engaged by higher-order cognitive tasks, including fronto-cerebellar, parietal-cerebellar, fronto-parietal, and cingulo-opercular networks (Beckmann, et al., 2005
; Fransson, 2006
; Dosenbach, et al., 2007
; Seeley, et al., 2007
). In sum, fMRI resting state research has found reproducible evidence for a “family” of 10 or more distinct networks engaged during rest (Beckmann et al., 2005
; De Luca et al., 2005
; Damoiseaux et al., 2006
; Calhoun et al., 2008a
). Resting state networks are also present during and modulated by cognitive task performance (where they are usually referred to as “default mode networks”; DMNs). Such circuits are more generally termed “temporally coherent networks” (TCN’s; Calhoun et al., 2008a
) and are robust, straightforwardly identified using ICA and can be consistently identified at rest and during cognitive tasks. As well as the “classic” DMN a bilateral temporal network is prominent.
The DMN is highly metabolically active, being responsible for approximately 80% of brain energy metabolism. It participates in organized baseline brain “idling” and maybe represent self-reflection, focus on internal stimuli, stream of consciousness or other activities (Gusnard et al., 2001
); certainly it diminishes during task-related behaviors (Raichle et al., 2001
) in a manner proportional to task difficulty (McKiernan et al., 2003
). In general, the more effortful the cognitive task, the more the classic resting state/default mode network activity diminishes during task engagement, (McKiernan et al., 2003
): in addition multiple “families” of TCN’s also show temporal and spatial modulation during cognitive tasks versus rest. This information was used by Garrity et al. (2007)
, who extracted default mode activity during performance of an auditory oddball task, and showed abnormalities in schizophrenia that correlated with both positive and negative illness symptoms. While in healthy subjects the network resonated slowly and regularly, this activity in schizophrenia was increased, more irregular and correlated with positive symptoms. DMN BOLD in schizophrenia was both over-and under-active within different regions, but the entire circuit appeared unable to stabilize itself in the default mode.
Other groups have also demonstrated significant differences between schizophrenia patients and controls using resting state or default mode data, (Liang et al., 2006
; Whitfield-Gabrieli et al., 2009
). Consistent with its status as a putative schizophrenia endophenotype, these data reveal abnormalities in unaffected relatives of schizophrenia subjects, (Whitfield-Gabrieli et al., 2009
) and are patterns are heritable ( Broyd et al., 2009
; Glahn et al., under review).
Combining information from separate TCNs is also useful. In a separate experiment examining diagnostic discrimination between schizophrenia, psychotic bipolar disorder and healthy controls, an approach incorporating data from both the classic default and temporal lobe modes derived from an AOD task using a leave-one-out approach, was able to achieve an average sensitivity and specificity of 90% and 95% respectively Calhoun et al. (2008b)
. This showed the utility of the default mode as a diagnostic classifier even when two psychotic groups were included in the analysis.
As discussed above, complex cognition arises from task-related, widely distributed groups or networks of brain regions (Fuster, 2006
). Functional connectivity analyses provide an opportunity to extend our knowledge regarding neural circuits. As discussed in Calhoun et al. in press
(accompanying article, this volume), the profile and strength of network-to-network influences, i.e. interactions across, rather than within networks, (“functional network connectivity”; (FNC) contains useful information. ICA of fMRI is well-suited to characterize multiple functional networks because by definition the brain regions in each component have the same profile of hemodynamic signal change. Demirci et al. (2009) and Jafri et al. (2008)
recently examined functional network connectivity in controls and schizophrenia patients during resting state alone or in addition to WM and attention tasks, to examine the weaker temporal relationships (such as lags) between circuits. Such studies not only found evidence for measurable, directed influences among large-scale distributed functional networks in controls, but also found that schizophrenia was characterized by widespread disruption, greater dependency, and greater variability of network inter-relationships, possibly reflecting cortical processing deficiencies. Schizophrenia subjects showed significantly higher correlations than controls among many of the dominant resting state networks (see additional details in Calhoun et al., in press
).
In sum, several different functional networks identified through ICA of BOLD activation appear to be important indicators of schizophrenia pathophysiology. As suggested by Mesulam (1998)
emerging neural network research indicates that these circuits are commonly engaged across many tasks in both schizophrenia and control groups, including networks subserving complex focused attention, “brain idling”, working memory/executive decision-making, set maintenance and language (1) prefrontal-parietal, (2) cingulate-opercular, (3) temporal lobe and (4) the classic “default mode” network). These circuits are focused around four major anatomic hubs already implicated in schizophrenia and suggest illness-related deficits might arise from abnormalities in the quality or strength of functional connections among major nodes. Earlier we discussed one of these networks, the prefrontal-parietal, extensively in the context of WM; thus these two apparently separate lines of research (complex “stress test” and “no task”) converge as can analysis methods, (Arfakanis et al., 2000
). Therefore, an overarching hypothesis is that the “disconnection syndrome” in schizophrenia represents miscommunication and/or disconnection between these key networks, which can be best understood through analytic approaches that determine how structural or functional connectivity abnormalities underlie the well-documented cognitive deficits or symptomatic syndromes.
Most of the presumed many schizophrenia risk genes remain unknown and may exert their effects epistatically. As functional MRI endophenotypes emerge, an obvious question is how to connect them to the complex patterns of emerging schizophrenia risk genes as the latter are identified (e.g. see Harrison and Weinberger, 2005
), especially using newer approaches such as genome-wide association studies (GWAS). Analyzing such large, complex data sets involving the millions of gene variants and hundreds of thousands of voxels typically involved in a GWAS/functional imaging study, can rapidly overwhelm any relevant signal (Pearlson, 2009
). This challenge has led to the development of new exploratory statistical techniques such as parallel independent component analysis (paraICA), (Liu et al., 2008
) for analyzing such high-dimensional, multimodal data. A recent paper (Liu et al., 2009a
,b
) used this algorithm to identify simultaneously independent components of imaging and genetic modalities and the relationships between them.
Parallel ICA is a variant of ICA designed for multimodality processing. It extracts components using an entropy term based on information theory to maximize independence (Bell and Sejnowski, 1995
) and enhances the interconnection by maximizing an inter-modality linkage function (Liu et al., 2009a
,b
), i.e. it extracts the intrinsic relationship between identified independent components from two distinct modalities based on higher-order statistics. The technique is readily used as an approach for revealing relationships between brain function and SNP groupings, i.e. to identify a combination of SNPs related to functional brain networks. This involves simultaneously solving three problems: revealing a set of specific independent brain functions, identifying independent SNP associations, and finding the correlative relationship (mutual information) between them Liu et al. (2008)
. The resulting components extracted from fMRI data can be interpreted as spatially distinct networks of brain regions expressing functional changes in different subjects to different degrees. For instance, the degree to which a given network is present may distinguish healthy subjects from schizophrenia patients. Similarly, components extracted from SNP data are distinct, independent, linear combinations of SNPs (“clusters” of functionally linked SNPs, likely representing SNPs with common interactions) that may affect certain genetic functionalities or phenotypes, or even clusters of physiologically interacting genes. Loading parameters (Liu et al., 2008
, 2009a
,b
) which express the association for every component with each subject are calculated.
As proof of principle, using data derived from an fMRI auditory oddball task in only 43 healthy controls and 20 schizophrenia subjects (all Caucasian) the paraICA approach was able to identify (Liu et al., 2009a
,b
) a fronto-parietal fMRI component that significantly separated schizophrenia patients from healthy controls, and an associated 10-SNP component that also significantly separated groups, that contained several known putative schizophrenia risk genes, including DISC1, CHRNA7 and the alpha-2 adrenergic receptor gene. Thus ParaICA seems to be a sensitive technique for dealing with gene/functional circuit interactions in medium-sized data sets.
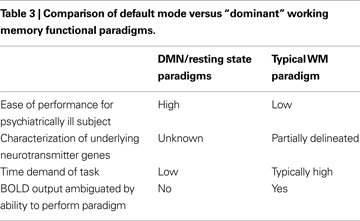
We have attempted to summarize briefly both where the field is currently with regard to endophenotype discovery and validation as well as related advances in model building, proceeding from the new genetic and neuroimaging tools available to the field. In this context, we contrast data derived from fMRI studies using two counterposed approaches; the dominant paradigm of “cognitive stress tests” based on cognitive tasks on which schizophrenia subjects are previously known perform poorly versus newer, resting state/default mode studies where cognitive effort is minimized, that represent conceptually different approaches to endophenotype discovery as summarized in Table 3
. Because of the wealth of prior experiments in animals and humans on WM paradigms, a large body of data exists on WM including major regions involved, relevant neurotransmitters, possible cell types, and whether major genes are known to play a role in the process. In contrast, the major “purpose”, if indeed there is one, for the default mode is still under active debate (for example see a detailed discussion in Buckner et al., 2008
), and no related genetic underpinning has yet been revealed. However the very specificity of detail regarding WM is both a strength and weakness; WM may yet prove to be only a particularly marked example of a more generalized process that characterizes the fundamental pathophysiology of schizophrenia. Abnormalities in the default mode or in the ability to switch between the default mode and effortful cognitive tasks such as WM may be of greater relevance. In any event both of the approaches we delineate identify brain patterns that meet many criteria for endophenotypes in SZ, as summarized previously in Table 1
. The gaps in that table show that not all endophenotypic criteria are met, because some needed data are not yet available. This presents an exciting challenge to researchers.
More generally, do functional neuroimaging studies perform better than other putative schizophrenia endophenotypes? No studies have yet sufficiently compared different endophenotypes in the same populations of patients, relatives and healthy controls to address this question, as well as clarifying their disease specificity, e.g. compared to psychotic bipolar disorder. Ultimately, understanding the genetic architecture of imaging endophenotypes is likely to prove extremely important in better comprehending what constitutes biological risk for schizophrenia. If schizophrenia proves to be a disorder of cortical connectivity and functional imaging intermediate phenotypes have a simpler genetic architecture than the full clinical disorder, uncovering gene interactions that underpin functional disconnections is a priority. In particular, finding genes influencing DMN/RS as suggested by Meyer-Lindenberg (2009) and discovering how such genes “build” functional circuits in both the normal brain as well as how these differ in risk-related variants in schizophrenia and at-risk individuals is crucial. Several recent large-scale studies of clustering patterns of multiple endophenotypes within and between categories of psychosis, such as Consortium on the Genetics of Schizophrenia (COGS; Braff et al., 2007
; Calkins et al., 2007
) and The Bipolar Schizophrenia Network on Intermediate Phenotypes (B-SNIP; Thaker, 2008
; Pearlson, 2009
) are now addressing these questions. In addition, the field needs to take better advantage of analytic tools of great power that have become available recently and that allow problems of the degree of complexity of schizophrenia pathophysiology to be analyzed adequately. These tools include approaches such as paraICA, in which multiple genes (and perhaps their epistatic interactions; Liu et al., 2009a
,b
) can be modeled and the gene clusters thereby identified studied subsequently with molecular pathway tools to uncover their collective interactions in cellular processes that likely underlie schizophrenia’s pathophysiology.
Other novel suggestions have been to combine multiple potential functional imaging intermediate phenotypes into “extended endophenotypes” as originally suggested by Prasad and Keshavan (2008)
for structural endophenotypes. One examples of this approach for the Temporal Lobe and Default Modes was discussed earlier (e.g. Calhoun et al., 2008b
). Other fruitful directions for future research should include more detailed exploration of the relationship between the WM and DMN circuits in schizophrenia- for example using FNC- this would likely provide useful information. Ultimately, dysfunction in both circuits may represent different examples of deficient cortical information processing (e.g. Callicott et al., 2003b
), or the two processes may prove to be unrelated and to originate in separate pathophysiological processes.
For the reasons summarized above, the next few years are likely to see a proliferation of publications on schizophrenia endophenotypes, and among these papers dealing with the genetics of functional imaging using the two major approaches we contrast will be undoubtedly strongly represented.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported in part by the National Institutes of Health (NIH), under grants R01MH077945, R37 MH43775 (MERIT Award) and MH074797 to Godfrey D. Pearlson: and 1 R01 EB 000840, 1 R01 EB 005846, and 1 R01 EB 006841 to Vince D. Calhoun.
Blokland, G. A., McMahon, K. L., Hoffman, J., Zhu, G., Meredith, M., Martin, N. G., Thompson, P. M., de Zubicaray, G. I., and Wright, M. J. (2008). Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: a twin fMRI study. Biol. Psychol. 79, 70–79.
Calkins, M. E., Dobie, D. J., Cadenhead, K. S., Olincy, A., Freedman, R., Green, M. F., Greenwood, T. A., Gur, R. E., Gur, R. C., Light, G. A., Mintz, J., Nuechterlein, K. H., Radant, A. D., Schork, N. J., Seidman, L. J., Siever, L. J., Silverman, J. M., Stone, W. S., Swerdlow, N. R., Tsuang, D. W., Tsuang, M. T., Turetsky, B. I., and Braff, D. L. (2007). The Consortium on the Genetics of Endophenotypes in Schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr. Bull. 33, 33–48.
Callicott, J. H., Ramsey, N. F., Tallent, K., Bertolino, A., Knable, M. B., Coppola, R., Goldberg, T., van Gelderen, P., Mattay, V. S., Frank, J. A., Moonen, C. T. W., and Weinberger, D. R. (1998). Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 18, 186–196.
Callicott, J. H., Straub, R. E., Pezawas, L., Egan, M. F., Mattay, V. S., Hariri, A. R., Verchinski, B. A., Meyer-Lindenberg, A., Balkissoon, R., Kolachana, B., Goldberg, T. E., and Weinberger, D. R. (2005). Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 102, 8627–8632.
Demirci, O., Stevens, M. C., Andreasen, N. C., Michael, A., Liu, J., White, T., Pearlson, G. D., Clark, V. P., and Calhoun, V. D. (2009). Investigation of relationships between fMRI brain networks in the spectral domain using ICA and Granger causality reveals distinct differences between schizophrenia patients and healthy controls. Neuroimage 46, 419–431.
Di Giorgio, A., Blasi, G., Sambataro, F., Rampino, A., Papazacharias, A., Gambi, F., Romano, R., Caforio, G., Rizzo, M., Latorre, V., Popolizio, T., Kolachana, B., Callicott, J. H., Nardini, M., Weinberger, D. R., and Bertolino, A. (2008). Association of the SerCys DISC1 polymorphism with human hippocampal formation gray matter and function during memory encoding. Eur. J. Neurosci. 28, 2129–2136.
Dosenbach, N. U., Fair, D. A., Miezin, F. M., Cohen, A. L., Wenger, K. K., Dosenbach, R. A., Fox, M. D., Snyder, A. Z., Vincent, J. L., Raichle, M. E., Schlaggar, B. L., and Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 104, 11073–11078.
Driesen, N. R., Leung, H. C., Calhoun, V. D., Constable, R. T., Gueorguieva, R., Hoffman, R., Skudlarski, P., Goldman-Rakic, P. S., and Krystal, J. H. (2008). Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol. Psychiatry 64, 1026–1034.
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., Zaitsev, E., Gold, B., Goldman, D., Dean, M., Lu, B., and Weinberger, D. R. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269.
McDonald, C., Marshall, N., Sham, P. C., Bullmore, E. T., Schulze, K., Chapple, B., Bramon, E., Filbey, F., Quraishi, S., Walshe, M., and Murray, R. M. (2006). Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am. J. Psychiatry 163, 478–487.
Ritsner, M. S., and Gottesman, I. I. (2009). Where do we stand in the quest for neuropsychiatric biomarkers and what next?, Chapter 1. In The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes, Volume 1: Neuropsychological Endophenotypes and Biomarkers, M. S. Ritsner, ed. (New York, Springer), pp. 3–17.
Thermenos, H. W., Seidman, L. J., Breiter, H., Goldstein, J. M., Goodman, J. M., Poldrack, R., Faraone, S. V., and Tsuang, M. T. (2004). Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: a pilot study. Biol. Psychiatry 55, 490–500.
Whitfield-Gabrieli, S., Thermenos, H. W., Milanovic, S., Tsuang, M. T., Faraone, S. V., McCarley, R. W., Shenton, M. E., Green, A. I., Nieto-Castanon, A., LaViolette, P., Wojcik, J., Gabrieli, J. D. E., and Seidman, L. J. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 106, 1279–1284.
Woodward, N. D., Waldie, B., Rogers, B., Tibbo, P., Seres, P., and Purdon, S. E. (2009). Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr. Res. 109, 182–190.