1
Croatian Institute for Brain Research, School of Medicine, University of Zagreb, Zagreb, Croatia
2
Department of Medical Biology, School of Medicine, University of Zagreb, Zagreb, Croatia
The purpose of this focused review is to present and discuss recent data on the changing organization of cerebral midline structures that support the growth and development of the largest commissure in humans, the corpus callosum. We will put an emphasis on the callosal growth during the period between 20 and 45 postconceptual weeks (PCW) and focus on the advantages of a correlated histological/magnetic resonance imaging (MRI) approach. The midline structures that mediate development of the corpus callosum in rodents, also mediate its early growth in humans. However, later phases of callosal growth in humans show additional medial transient structures: grooves made up of callosal septa and the subcallosal zone. These modular (septa) and laminar (subcallosal zone) structures enable the growth of axons along the ventral callosal tier after 18 PCW, during the rapid increase in size of the callosal midsagittal cross-section area. Glial fibrillary acidic protein positive cells, neurons, guidance molecule semaphorin3A in cells and extracellular matrix (ECM), and chondroitin sulfate proteoglycan in the ECM have been identified along the ventral callosal tier in the protruding septa and subcallosal zone. Postmortem MRI at 3 T can demonstrate transient structures based on higher water content in ECM, and give us the possibility to follow the growth of the corpus callosum in vivo, due to the characteristic MR signal. Knowledge about structural properties of midline morphogenetic structures may facilitate analysis of the development of interhemispheric connections in the normal and abnormal fetal human brain.
To accomplish complex tasks, mammals require coordinated brain activity, based on precise and efficient connections between the two hemispheres. These connections consist of axons that traverse the telencephalic midline, principally in three commissural tracts: the corpus callosum, the hippocampal commissure and the anterior commissure. Among these, the corpus callosum is the most voluminous fiber tract, and in the human species reaches its maximum complexity and size relative to brain volume (Gazzaniga, 2000
). Anatomical studies in experimental rodents demonstrated that the majority of contralaterally projecting (callosal) neurons are located in cortical layers II/III and layer V (Innocenti and Price, 2005
), while in the primate brain fibers of the corpus callosum predominantly originate from layer III pyramidal neurons of the neocortex (Mrzljak et al., 1988
; Schwartz and Goldman-Rakic, 1991
; Schwartz et al., 1991
). Axons of the callosal neurons elongate to the intermediate zone, then navigate medially through a well-defined pathway along the medial wall of the ipsilateral ventricle, cross the midline, grow further into the contralateral hemisphere towards the target region and area (usually homotopic), and finally enter the appropriate cortical layer to establish functional connections. Members of the Netrin, Slit, Semaphorin, Ephrin and Wnt families of guidance molecules and their receptors, coordinate this extremely demanding navigation. As secreted and diffusible molecules, they have long-range effect on the growth cone and axons, or as molecules attached to the cellular membranes or extracellular matrix (ECM), they have short-range effects (see reviews Judas et al., 2003
; Lindwall et al., 2007
; Plachez and Richards, 2005
). Recent findings suggest that the axis of axon elongation is determined even prior to axon outgrowth by the manner in which Netrin, Slit and Wnt receptors are localized within the neuron (Killeen and Sybingco, 2008
). Expression of these molecules is regulated by both intrinsic cell-autonomous factors (transcription factors that coordinate receptor expression and signaling at the growth cone membrane) and by extrinsic factors in the extracellular environment.
Several well-known developmental mechanisms, such as guidance by pioneering axons, guidance by pre-existing axonal tracts and guidance by cellular structures have been ascribed to various morphogenetic zones involved in the complicated pathfinding during the formation of commissures in a mammalian brain. However, these different morphogenetic zones have some principal properties in common: (1) strategic location, (2) sequential appearance and dissolution, i.e. particular developmental window, (3) modular or laminar appearance, (4) versatile expression of guidance cues and (5) abundance of ECM. Disturbances in finely tuned expression of guidance and ECM molecules in the morphogenetic zones might cause structural or functional anomalies ranging from subtle cognitive impairment to severe developmental abnormalities, including dysgenesis and agenesis of the corpus callosum and other commissures (reviewed by Paul et al., 2007
; Richards et al., 2004
).
In this review, we discuss dynamic changes of the properties of morphogenetic zones related to corpus callosum growth, revealed by a new application of classical histological methods, molecular biology techniques and advanced high-resolution brain imaging, focusing on the human midline and its specificities.
As soon as they arise from the soma of future cortical neurons, the predetermined cortical efferents grow toward the intermediate zone guided by gradients of different guidance molecules (for review on the spatio-temporal distribution of guidance cues in the transient embryonic and fetal zones see Judas et al., 2003
). When they reach the intermediate zone, the axonal populations have to decide to grow medially around the ipsilateral ventricle as future callosal axons, or to grow laterally toward the internal capsule as long subcortically projecting axons, in both cases avoiding proliferative zones which express repelling cues, e.g., semaphorin3A (SEMA3A) (Bagnard et al., 1998
). Data regarding this decision point in human callosal formation are still missing, but exuberant axonal bifurcations have been reported in mice. Bifurcations are supposed to be a developmental mechanism for some cortical neurons to choose elongation toward the midline after cutting off the lateral branch if it never reaches a viable target (Garcez et al., 2007
). Moreover, it has recently been demonstrated in mice that the expression of the guidance cue neuropilin1 (Npn1, a receptor for SEMA3A) by the growing callosal axons, has a critical role in the choice of tangential extension toward the midline (Hatanaka et al., 2009
).
The early growth of future callosal axons in humans and the subsequent morphogenesis of the callosum were firstly demonstrated by histological methods and described in the classical embryological studies (for review see Rakic and Yakovlev, 1968
). Prior to corpus callosum formation, at 11 postconceptual weeks (PCW), a new structure designated as massa commissuralis is rapidly formed after the fusion of median groove banks above the septal area in the so called “commissural plate of Hochstetter” (Rakic and Yakovlev, 1968
). The first axons, named pioneering, approach and penetrate the massa commissuralis at the mediosagittal plane after 11 PCW (Rakic and Yakovlev, 1968
). Therefore, the medially positioned massa commisuralis is probably the first midline structure that expresses specific molecules and morphogenes for guidance and nurture of pioneering callosal axons in humans. Moreover, it has recently been shown that a portion of developing callosal axons at 17 PCW expresses Npn1, and that a considerable number of those originate from the cingulate cortex (Ren et al., 2006
). These axons most likely represent the pioneering axons in human callosal development, considering the newly established central role of Npn1 in the development of pioneer projections from the cingulate cortex of mice (Piper et al., 2009
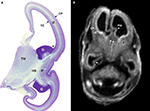
). Correlation of in vitro magnetic resonance imaging (MRI) and histological analysis of the developing human cerebrum revealed that the commissural plate, as well as other transient fetal zones, can be visualized on T1-weighted images already at 10 PCW (Figure 1
, Rados et al., 2006
). The commissural plate is then at the onset of its development and visibility on MRI scans as a thickened dorsal part of the telencephalon impar (Figure 1
B double arrows, Rados et al., 2006
). The callosal fibers that penetrate massa commissuralis and form the callosal plate, can be demonstrated by histological methods at 12–13 PCW (Rakic and Yakovlev, 1968
), while the earliest stage of their visualization by diffusion tensor imaging (DTI) is at 14–15 PCW (Huang et al., 2009
; Ren et al., 2006
). Some reports have suggested that the first axons are the ones that will form the rostrum, the genu and the body (Ren et al., 2006
), while others suggest that the callosum will grow in both anterior and posterior directions, with a more prominent anterior growth (Huang et al., 2009
).
Figure 1. Semi-horizontal Nissl-stained slide (A) and T1-weighted MRI slice (B) through the fetal telencephalon at 11.5 PCW (A) and 12.5 PCW (B). The cerebral wall consists of the ventricular zone (VZ), the intermediate zone (IZ) and the cortical plate (CP). Trilaminar organization of the cerebral wall, the ganglionic eminence with developing caudate and putamen, and the hippocampal anlage (arrowhead Hip) are all clearly visible on both histological and MRI sections. The two fornix bundles merge toward the septal area and contribute to the triangular shape of the telencephalon impar in MRI scans. Double arrows in (B) point to the commissural plate. C, caudate nucleus; CP, cortical plate; G, ganglionic eminence; Hip, hippocampus; HS, hemispheric stalk; IZ, intermediate zone; P, putamen; TH, thalamus; VZ, ventricular zone. Modified and reproduced with permission from Rados et al. (2006)
. Copyright Elsevier Ltd.
With the formation of the callosal plate, several morphogenetic zones appear along the midline and subsequently develop into transient cellular structures: the midline sling, the glial wedge and the glia of indusium griseum (indusial glia). These developmental structures were first recognized and described in morphological studies of experimental animal models (Silver et al., 1982
, 1993
). Later they were further explored in mice by modern molecular methods, and for each structure a critical role in the morphogenesis of the corpus callosum has been established (Shen et al., 2006
; Shu and Richards, 2001
; Shu et al., 2003a
,b
,c
; Smith et al., 2006
; Tole et al., 2006
). Recently, histological and correlated histological/MRI studies demonstrated that these transient structures are also present during the period of early development of the human forebrain midline (Lent et al., 2005
; Ren et al., 2006
), indicating conservation of developmental mechanisms and structures during mammalian evolution. These morphogenetic structures situated in strategic locations have overlapping developmental windows between 13 and 20 PCW (Lent et al., 2005
; Ren et al., 2006
).
The midline sling was originally described as a thin concave lamina consisting of migrating glia-like cells that tightly underline the ventral surface of the developing corpus callosum (Silver et al., 1982
, 1993
). It was later proven that, at least in mice, a substantial portion of cells that forms the sling are neurons (Shu et al., 2003b
). Neuronal nuclear antigen (NeuN), calretinin and glial fibrillary acidic protein (GFAP) have been demonstrated in the human midline sling, indicating that it also consists of neuronal and glial cells (Ren et al., 2006
). The first clearly NeuN positive reactivity was shown to be located paramedially on the corticoseptal border at 13 PCW, but at a later stage (15 PCW) numerous neurons were observed more medially (Ren et al., 2006
), suggesting that this population of cells migrates toward the midline. The origin of the cells that compose the midline sling in humans is complex and not clearly understood. However, one can speculate that similarly to the sling-cells in mice (Shu et al., 2003b
), they are coming from the adjacent subventricular zone (Ren et al., 2006
). Although, the pioneer callosal axons of mice (Ozaki and Wahlsten, 1998
; Rash and Richards, 2001
) cross the midline before the development of the sling at embryonic day (E) 17 (Silver et al., 1982
), prenatal experimental lesioning causes a failure in corpus callosum formation, thus confirming the significance of midline sling in this developmental point (Silver and Ogawa, 1983
).
The glial wedge at the gross structural level is described as a bilaterally symmetrical modular structure located at the corticoseptal boundary between the cingulate cortex (dorsal telencephalon) and the septum (ventral telencephalon). The corticoseptal boundary is genetically defined (Shen et al., 2006
), and its correct dorsoventral position is critical for the formation of both the glial wedge and the corpus callosum. In the human brain, the glial wedge is present at 14 PCW, flanking the ventral side of the corpus callosum. It is densely populated by cells that are positive for nuclear factor Ia (Nfia), vimentin and GFAP. These cells are in continuity with the radial glial cells of the ventricular zone of the cerebral wall (Lent et al., 2005
; Ren et al., 2006
). Differentiation of the glial wedge cells into astrocytes begin at 14 PCW, earlier than in the rest of the cortical wall, and lasts at least through the first half of gestation (Rezaie et al., 2003
). Experimental work in mice, has pointed out that guidance by the glial wedge occurs through SLIT–ROBO and WNT–RYK signaling (Andrews et al., 2006
; Keeble et al., 2006
; Shu and Richards, 2001
; Shu et al., 2003d
).
In the human brain, the indusial glia begins to develop at about 14 PCW, when discernible Nfia (Ren et al., 2006
), GFAP (Lent et al., 2005
; Ren et al., 2006
) and vimentin (Lent et al., 2005
) expression has been shown dorsally to the developing corpus callosum. The importance of indusial glia for callosal development has been convincingly demonstrated by Nfia- and Nfib-knockout mice. These have significantly reduced or absent indusial glia and glial wedge, and fail to form the corpus callosum (Shu et al., 2003a
; Steele-Perkins et al., 2005
). In the conditional knockout mice for fibroblast growth factor receptor 1/GFAP (Fgfr1/Gfap), it has been shown that indusial glia originates from ventricular radial glial cells (Fgfr1 gene is critical for dorsally directed migration of radial glial cells in the midline) (Smith et al., 2006
). When Fgfr1 is knocked out earlier in development, all midline glial structures that derive from the corticoseptal boundary and corpus callosum, fail to develop (Tole et al., 2006
).
The list of guidance molecules, transcription factors and morphogens involved in corpus callosum formation in mice and humans, which are expressed either in the midline structures or by growing callosal axons, is continuously expanding (see review articles Judas et al., 2003
; Lindwall et al., 2007
; Plachez and Richards, 2005
; Richards et al., 2004
). However, evidence for expression of these molecules in the human brain midline is still very scanty. Northern blot analysis of total RNA and mRNA for Netrin1, its receptor deleted in colon cancer, Slit1, Slit2 and Slit3, their receptors Robo1 and Robo2, and the transcription factors Nfia and Emx1, confirms that these nine genes are expressed in the human forebrain at 17 PCW, and that Netrin1, Slit2 and Slit3 have increased expression in ventrocaudal regions of the forebrain, compared to the dorsal neocortex (Ren et al., 2006
). The most informative part of the study was demonstration of the presence of Npn1 in axons of the human corpus callosum at 17 PCW (Ren et al., 2006
).
Studies in experimental rodents have demonstrated that ECM molecules such as laminin, fibronectin, proteoglycan NG2, heparan and chondroitin sulfate proteoglycans play an important role in the guidance of commissural axons (Braga-de-Souza and Lent, 2004
; Inatani et al., 2003
; Yang et al., 2006
). So far, in the human brain only tenascin-C was convincingly shown to be present dorsally and ventrally to the corpus callosum until 20 PCW (Lent et al., 2005
). ECM molecules rather than just constituting a structural scaffold, interact with guidance cues and can critically modulate axonal guidance function (reviewed by de Wit and Verhaagen, 2007
; Kleene and Schachner 2004
).
Taken together, studies on human brain development have confirmed that developmental mechanisms governing the early formation of the forebrain midline and corpus callosum are indeed very similar to, if not the same as those described in experimental rodents. However, the human fetal brain is significantly larger than the brain of prenatal rodents and the distances the growing commissural axons have to cover are notably longer in every segment of their complex journey. Therefore, it is reasonable that commissural connections in humans require a longer period of contemporaneous persistence of supporting midline structures and probably additional morphogenetic zones.
There are only a few histological studies describing the later stages of corpus callosum development in humans. In fetuses at 18–20 PCW, it is a well-developed fibrillar structure, which in coronal planes, runs transversally toward the midline and after crossing curves along the roof and lateral wall of the lateral cerebral ventricle. In the midsagittal plane, the outlines of the major callosal parts (genu, body and splenium) are clearly visible and assume the same shape and position as in the adult brain, with the exception of being much smaller in their rostrocaudal extent and thickness (Rakic and Yakovlev, 1968
). DTI studies of the fetal brain at 19 PCW show the callosal radiation with its typical shape, similar to the one seen in neonates, with axons from all parts of the callosum forming a mohawk-shaped structure (Huang et al., 2009
). At this stage the callosal cross-sectional area is only 5% of the size seen in a 5-year-old infant. At the neonatal stage, just before apparent myelinization of the callosum starts, it is half the size (Huang et al., 2006
).
Beside the early midline structures, a morphogenetic role in formation of the human corpus callosum during the second half of gestation has been described for two additional zones: callosal septa (Jovanov-Milošević et al., 2006
) and subcallosal zone (SCZ) (Kostović et al., 2002
). These two midline structures jointly form “grooves” in which callosal bundles are laid down during the second half of gestation.
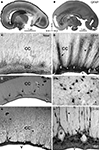
Postmortem analysis of the corpus callosum (age range from the 18 PCW to adult), immunostained for GFAP, NeuN and chondroitin sulfate proteoglycan (CS-56), in addition to classical histological methods, revealed the existence of modular cellular structures; namely, the callosal septa, which are most prominent during the second half of gestation (Figure 2
) (Jovanov-Milošević et al., 2006
). Callosal septa of variable sizes radially invade the corpus callosum from its ventral surface, dividing it into irregular segments (Figures 2
A,B). In horizontal sections, septa appear as thin railway slippers along the anteroposterior axis of the corpus callosum. In midsagittal sections, the callosal septa appear as radial striations wider in the ventral portion. In addition, thinner striations, one to three cell rows wide, are evenly spaced between the larger septa (Figures 2
G,H, arrowheads). Such modular structure and segmentation of the corpus callosum is visible in illustrations of midsagittal sections of the developing monkey (Killackey and Chalupa, 1986
) and human brain (Bayer and Altman, 2005a
,b
; Rakic and Zecevic, 2003
). However, callosal septa have not been explicitly described, and their importance in the protracted development of the corpus callosum in primates has been essentially overlooked. The reasons why the importance of these structures was not perceived in recent studies of the human midline might lie in the fact that the septa are not discernible in coronal sections through the corpus callosum, and the fact that the latest fetal age examined in these studies was 20 PCW.
Figure 2. Parasagittal (A) and midsagittal (B) sections of the human fetal brain showing the transient cellular structures callosal septa at 21 (A), 24 (E–H) and 25 (B–D) PCW stained with Nissl (A,C), glial fibrillary acidic protein (GFAP) (B,D), neuronal-specific nuclear protein (NeuN) (E,F) and chondroitin sulfate proteoglycan (CS-56) (G,H) immunocytochemistry. Framed areas in (A) and (B) are enlarged correspondingly in (C) and (D); (F) and (H) represent high magnification of callosal septa shown in (E) and (G). Arrows point at thicker callosal septa, arrowheads at striations. s, callosal septa; CC, corpus callosum; SCZ, subcallosal zone; V, cerebral ventricle; a, anterior; p, posterior; d, dorsal. Modified and reproduced with permission from Jovanov-Milošević et al. (2006)
. Copyright Collegium Antropologicum.
During the developmental window of 18–34 PCW, the number of callosal septa is individually variable. Usually 15–20 thicker and longer septa and numerous smaller septa are unevenly distributed along the anteroposterior axis (Figures 2
A–D). In the genu and the anterior part of the callosal body (Figure 2
B, framed area), the septa are more numerous and more regularly spaced (Figure 2
D, arrow). In the rest of the callosal body, their number declines, while in the splenium it increases again, but the septa are still less numerous and less prominent there, in comparison with the anterior portion of the callosum. At the cellular level, the callosal septa contain: GFAP reactive meshwork, NeuN positive neurons, CS-56 immunoreactive ECM (Figure 2
, Jovanov-Milošević et al., 2006
) and expression of the guidance molecule SEMA3A in cells and ECM (Judas et al., 2005
). The intensive GFAP staining of septa is associated with perivascular astrocyte elements, fine glial fibers and scattered GFAP positive astrocytes. The ventral, wider portion of the septa has a larger number of neurons (Figures 2
E,F) as well as a higher content of chondroitin sulfate proteoglycan (Figures 2
G,2
H). At midgestation, SEMA3A is expressed in septa of the anterior third of the callosum and above the fornix (Figure 3
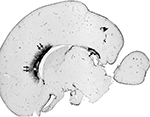
, Judas et al., 2005
). It is important to note that chondroitin sulfate proteoglycan in the ECM plays an important role in the cellular localization of SEMA3A, and their interaction can modulate the biological activity of this guidance molecule (de Wit et al., 2005
).
Figure 3. SEMA3A immunoreactivity in human brain midline at 20 PCW is restricted to ventral area of anterior callosal portions, where it is located in both cell bodies and the extracellular matrix. The SEMA3A is prominent in callosal grooves: in septa (double arrows) and in the subcallosal zone (asterisk). In addition, strong SEMA3A immunoreactivity is visible dorsally to the fornix system (arrow). Modified and reproduced with permission from Judas et al. (2005)
. Copyright American Society of Neuroradiology.
The callosal septa have not been shown by conventional MRI, yet the abundance of ECM and proteoglycans in septa, as well as their radial orientation between callosal bundles, should influence the MRI signal in diffusion-weighted imaging (DWI) of the human fetal brain. In vivo DWI of infants born between the ages of 25 and 34 PCW, with some having follow-up scans at term equivalent age, showed lower fractional anisotropy (FA) values in the genu than in splenium. Decreased FA values generally indicate a less coherent parallel organization of axons, which in this case may be due to the presence of septa in the genu. The study also showed higher apparent diffusion in the genu, indicating higher water content and smaller fiber density (Partridge et al., 2004
), possibly due to chondroitin sulfate proteoglycan in the callosal septa and their remnants. In utero DWI of fetal white matter between 18 and 37 PCW also showed lower FA values in the genu. As the authors themselves suggested, this might be a consequence of a specific geometric microstructure of the callosal septa. The predominance of radially oriented septa within the genu would decrease its FA value (Kasprian et al., 2008
).
The ventral part of the callosal septa is in continuation with the SCZ, which like a thin lamina occupies the median and paramedian territories situated between the developing corpus callosum (dorsally) and the fornix bundles (ventrally) (Kostović et al., 2002
). The cellular composition of the SCZ (revealed by acetylcholinesterase histochemistry and Golgi staining) is characterized by area-specific neuron-like cells with long and wavy processes, large glia-like cells, maturing neurons, migratory-like neurons and radial glial cells (Figure 4
), with a difference in distribution between the medial (nucleus septohippocampalis) and lateral portion (allocortical counterpart of the subventricular zone). In fetuses at midgestation, the lateral paramedian portions of the subcallosal region gradually transforms into the subventricular zone of the dorsal neocortical telencephalic wall, both described in detail by Zecevic group (Zecevic et al., 2005
). Some of the cells of SCZ also express SEMA3A (Judas et al., 2005
).
Figure 4. Different types of cells in the subcallosal region of a 22-PCW (A) and a 28-PCW human fetus (B), as reconstructed by Neurolucida software. The subcallosal zone (SCZ) at both stages contain radial glial cells (B10), glia-like cells (A1, A3), migratory-like neurons (A7), immature polymorphic neurons (A6, 8, 9, 10, 12, 14,15 and B2, 4, 6, 7, 12), and region-specific large neuron-like cells with long wavy dendrites oriented predominantly towards the corpus callosum (CC), partly penetrating it (A2, 4, 5, 13 and B1, 3, 5, 8, 9, 11). Note that processes of large neuron-like cells seem to be less profusely branched in the older specimen. Scale bar: 50 μm. Modified and reproduced with permission from Kostović et al. (2002)
. Copyright S. Karger AG, Basel.
During the early postnatal period, the callosal septa become thinner and shorter, lose their neuronal and chondroitin sulfate proteoglycan content. At the same time, the number of cells in the SCZ decreases.
Several lines of evidence support the morphogenetic role of the callosal grooves. The period of developmental peaks of the callosal septa and SCZ (between 18 and 34 PCW) corresponds to the period of intensive growth of callosal fibers (Huang et al., 2006
). The septa are appropriately shaped to support the transverse growth of callosal fibers, similarly to the midline sling during early callosal development in mice and humans (Ren et al., 2006
; Shu et al., 2003b
; Silver et al., 1982
). The complexity and preferential orientation of the processes of subcallosal cells, as well as those of cells in the septa, suggest that they may be involved in the guidance of callosal axons. The cells of the septohippocampal continuum may have a pivotal role in the bidirectional guidance of fornix fibers. The cells of the callosal septa produce the axonal guidance molecule SEMA3A (Judas et al., 2005
), shown to be necessary for active guidance of callosal axons across the midline towards the opposite hemisphere in mice (Piper et al., 2009
). If the Npn1 expression, which was demonstrated at 17 PCW (Ren et al., 2006
), still persists during the second half of gestation, it is reasonable to presume that it would continue to interact with its ligand, such as SEMA3A. Finally, the ECM and chondroitin sulfate proteoglycan in septa and SCZ can interact with guidance molecules and serve to guide growing callosal axons along the ventral callosal moiety during the second half of gestation. A dual developmental role has also been shown for the chondroitin sulfate proteoglycan: in the subplate it is a favorable substrate for growth of thalamocortical axons, but at the same time, it is an inhibitory substrate for developing cortical efferents forcing them to grow in a chondroitin sulfate proteoglycan negative intermediate zone (Bicknese et al., 1994
; Miller et al., 1995
). In that respect, proteoglycans in the brain midline may guide the growth of callosal axons along the ventral callosal tier, similarly to the role of subplate-proteoglycans in the growth of thalamocortical connections. Or they may act as an unsuitable growing substrate, confining callosal axons to the mainstream of growth (Jovanov-Milošević et al., 2006
; Judas et al., 2005
; Lent et al., 2005
). An additional role of the ECM is maintaining diffusible guidance molecules within callosal grooves (Jovanov-Milošević et al., 2006
).
The corpus callosum of adult primates consists of “segments” which contain topographically segregated callosal fibers for a given cortical area (Moses et al., 2000
; Pandya et al., 1971
; Witelson, 1989
). Thus, callosal septa with their topographic arrangement, shape and content most likely provide a basis for topographically ordered commissural projections from one hemisphere to another, or at least maintain the topographical relationship during development. Confirmation of this suggestion can be found in the fact that the basic topography and terminal field patterns of callosal projections in monkey brain are established already by E133, well before birth (Dehay et al., 1988
; Killackey and Chalupa, 1986
; Schwartz and Goldman-Rakic, 1990
, 1991
). It should be noted that in mice, newly added axons grow along the ventral tier of the corpus callosum (Ozaki and Wahlsten, 1992
). In addition, tractography studies in humans showed an evident dorsoventral fiber distribution in the adult human brain, with earlier developed medial cortical areas sending their fibers dorsally through the corpus callosum, while later developed laterodorsal cortical regions send them ventrally (Tovar-Moll et al., 2007
). In this respect, callosal septa and SCZ with their abundance of ECM and guidance molecules along the ventral aspect of the corpus callosum are strategically located for influencing the growth of callosal axons.
One of the principal mechanisms of cortical development is an overproduction of axons, axonal branches and synapses, the so-called developmental exuberance, which is followed by a subsequent selection and refinement based on adequate target region recognition and activity of the functional connections (reviewed by Innocenti and Price, 2005
). The corpus callosum is a pivotal example for such developmental exuberance, since the number of callosal axons in monkeys at E165, exceeds the number of callosal axons present in the adult by at least 3.5 times (LaMantia and Rakic, 1990a
,b
). Thus, the later morphogenesis of the corpus callosum is even more complex due to the processes of retraction of exuberant callosal fibers. In the primate brain, this continues during the first three postnatal months (LaMantia and Rakic, 1990a
). In the human brain, decrease in size of midsagittal cross-sectional area was observed after 32 PCW and this lasts to the second postnatal month. This suggests the beginning of a retraction of exuberant callosal axons (Clarke et al., 1989
), concomitantly with the resolution of waiting compartments (Kostovic and Rakic, 1990
) and the resolution of the majority of the callosal septa (Jovanov-Milošević et al., 2006
). The remnants of septa that continue for some time after birth may help in the process of withdrawal of exuberant callosal axons, since these structures and processes temporally overlap.
In addition, the septa may also provide corridors for migration of later-born neurons, since thick callosal bundles on the roof of the lateral ventricle represent a structural barrier. The groups of neurons originating from the subventricular zone may use glial fibers in the callosal septa to “climb”, while guidance molecules and ECM facilitate migration towards the cortex (Jovanov-Milošević et al., 2006
).
Recent progress in gene targeting methods, advances in axonal tracing, and high-resolution MRI techniques have revealed the morphogenetic zones and their role in guiding callosal axons across the midline. Although, the corpus callosum at midgestation assumes a shape and position not essentially different from that in the adult brain, it is still far from its definitive rostrocaudal extent and thickness (Huang et al., 2006
; Rakic and Yakovlev, 1968
). For each forebrain connectivity system, the growth of fibers (extension, accumulation and ingrowth) may last from 4 to 8 weeks with significant overlap among systems, but also with regional differences in timing, of up to 2 weeks (Kostovic and Jovanov-Milošević, 2006
). In the developing brain, all histogenetic events (neurogenesis, gliogenesis, migration, cell differentiation, axonal extension and synaptogenesis) proceed within laminar or modular compartments or zones. These do not have an equivalent in the adult brain (Kostovic et al., 2002
; Rakic et al., 2004
). Therefore, different structures and processes have to be interpreted in a precisely defined spatial and temporal context. In that respect, it is important to note that in humans the complex event of interhemispheric integration through the corpus callosum continues throughout gestation and well after birth. The second half of gestation and the early neonatal period are important for the multiple-fold increase of callosal axonal number, selection of functional axons, withdrawal of exuberant axons and targeting of the region, area, layer and cells of the cortical plate. Insight into the organization of the morphogenetic zones involved in development of cortico-cortical pathways is a prerequisite for the studies of preterm and term-born infants in normal development and pathological conditions. It has been previously suggested that in preterm infants the periventricular areas are vulnerable, but also show vigorous structural plasticity due to the abundance of ECM and guidance molecules, in addition to a “waiting” position of fibers (Judas et al., 2005
). Analogously, callosal septa with their content of ECM and guidance molecules give the callosum a potential for structural plasticity in infants when their development is disturbed prenatally. Contributing to that idea are the known adverse effects of preterm birth on cross-sectional callosal size, measured on structural MRI. Differences in size have been particularly notable in the posterior callosal portions (Nosarti et al., 2004
), where the septa are not so prominent. It is not yet clear whether defects in callosal septa formation can effect corpus callosum size and contribute to callosal hypoplasia in humans. MRI parameters such as FA may provide valuable information for prognostication and therapy management in prenatal brain injury, since neurodevelopmental impairments in infants born preterm are related to microstructural abnormalities in specific regions of the corpus callosum (Counsell et al., 2008
). Therefore, the knowledge of the sequence of events and the composition of developing forebrain midline enables a more reliable understanding of normative parameters necessary for studies of structural plasticity after perinatal brain injury.
Considering the clinical significance of the corpus callosum (over 50 different syndromes display dysgenesis of the corpus callosum and over 40 genes are linked to these anomalies, reviewed by Richards et al., 2004
), its importance for cognitive functions (Gazzaniga, 2000
; Teicher et al., 2004
) and its frequent lesioning in the perinatal period (Stewart et al., 1999
), we would strongly encourage long-term studies of the formation of commissural pathways in humans.
The authors declare that the research presented in this paper was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work is supported by MSES 108-1081870-1876 and UKF1B 06/07 grants to IK.
Counsell, S. J., Edwards, A. D., Chew, A. T., Anjari, M., Dyet, L. E., Srinivasan, L., Boardman, J. P., Allsop, J. M., Hajnal, J. V., Rutherford, M. A., and Cowan, F. M. (2008). Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 131, 3201–3208.