What can tracking fluctuations in dozens of sensory neurons tell about selective attention?
- 1 Department of Physics, Yale University, New Haven, CT, USA
- 2 Department of Neurobiology and Kavli Institute for Neuroscience, Yale University School of Medicine, New Haven, CT, USA
A commentary on
A neuronal population measure of attention predicts behavioral performance on individual trials
by Cohen, M. R., and Maunsell, J. H. R. (2010). J. Neurosci. 30, 15241–15253.
The brain possesses limited resources and utilizes selective attention as the mechanism to manage the massive influx of sensory information into the cortex. Selective attention strengthens the impact of behaviorally relevant information and diminishes distractions from irrelevant inputs. For instance, in visual discrimination or detection tasks, proper allocation of attention improves performance and shortens response times. At the neural level, there are many different effects of attention on the response of sensory neurons: receptive field shrinkages, modulation of neural synchronization and mean activity, variability reduction, interneuronal decorrelations, and more (Reynolds and Chelazzi, 2004). Yet few experiments have attempted to determine how attentional correlates subserve behavioral benefits (Womelsdorf et al., 2006).
Answering the question “How does attentional correlate ‘X’ subserve behavioral benefits?” requires reliable measurement of the attentional correlate on the timescale of behavioral changes. However, the high variability that is present in the activity of sensory neurons makes it difficult to observe a net effect of most attentional measures on a single-trial basis. Most of the experimental evidence is based on averaging activity across trials so that the signal-to-noise ratio increases and the attentional effect becomes significant. Such averaging presents strong limitations. It cannot be used to evaluate whether the attentional state changes within a given attentional condition, and to what extent attentional fluctuations, if they exist, covary with behavior on a trial-by-trial basis.
A recent study by Cohen and Maunsell (2010) has opened the door to analyzing attentional fluctuations by successfully measuring the amount of attentional allocation with single-trial temporal resolution. While monkeys performed an orientation change detection task, dozens of neurons were simultaneously recorded in both hemispheres of area V4. The monkey fixated a central point and one stimulus was repeatedly presented in each hemifield. When the orientation of one stimulus changed, the monkey was rewarded for making a saccade to it. The locus of spatial attention was manipulated in blocks of trials. At the beginning of each block, a cue indicated the hemifield in which 80% of orientation changes would occur. As expected, performance in detecting small orientation changes was improved by spatial attention: for a given level of performance, a smaller change in orientation was required in the cued compared to the uncued hemifield (7.6° difference in average).
Cohen and Maunsell devised a trial-based measure that extended the typical single-neuron measure of attentional modulation. For each correct trial, the activity from all recorded neurons during the previous stimulus (i.e., the stimulus preceding the orientation change) was represented as a point in a multi-dimensional space, where each dimension encoded the activity of a single neuron. Next, an “attention axis” was defined as the line connecting the centroids of two clusters, each associated with one attentional condition (e.g., attention to the left hemifield). The projection of each point on the attention axis represented a measure of the attentional modulation in that trial. Projections from error trials tended toward the opposite attentional condition, indicating that the attentional modulation was less biased.
These measurements showed that within a trial, attention during one stimulus presentation correlated with attention during the subsequent presentation. This explained why performance, ranging from nearly 0 to 70% correct trials within an attentional condition, covaried on a single-trial basis with the attentional state during the previous stimulus. For example, a high attentional allocation during the previous stimulus was associated with a good discrimination along the attention axis, which mostly persisted into the change stimulus, and eventually facilitated change detection. The highly predictive power of the attentional measure relied on only few dozens of neurons, which demonstrated the potential of the procedure.
A remarkable and unanticipated finding of the Cohen and Maunsell (2010) study was that attentional fluctuations in each hemisphere appear to be independent. When the population was divided into two by hemisphere, the projections for the two separate hemispheres were not significantly correlated. This result has potential implications for the nature of the neural circuits serving as source of the attentional signal. In their view, this lack of correlation in V4 challenged the concept of a unified attentional “spotlight” that can only be directed to one location at a time, on the grounds that an attentional spotlight would induce an anticorrelation between the population projections of the two V4 hemispheres. Instead, they suggested that attention can be flexibly distributed across both hemifields and instantiated by separate neural populations with independent fluctuations.
However, this conclusion does not necessarily follow from observing uncorrelated attentional fluctuations in V4. For concreteness, we present two alternative scenarios based on a single source of attention. Critically, the viability of each alternative depends on the magnitude of attentional modulation in the less-attended hemisphere, which the authors could not measure by task design. Since only the difference in attention between the two locations could be measured, it is not known whether the strength of the top-down attentional signal to the less-attended hemisphere was moderate but significant or minimal. The strength of this signal reflects the neural activity pattern in the source area of attention (Figure 1). Assuming that the source of attention is described by Figure 1A, no significant correlations in the source area could be found if the separation of the neurons encoding the locations of both stimuli were larger than the footprint of recurrent synaptic inputs. Note that importantly, correlations could arise once the separation fell within these footprints. Assuming that the source of attention is described by Figure 1B, the activity of neurons encoding the less-attended location in the source area is very low. Suppression from the neurons encoding the attended location may exist, e.g., as a result of global inhibition. However, even if that anticorrelation exists in the source area, it would not be measurable downstream in the activity of V4 neurons because attentional modulations are negligible.
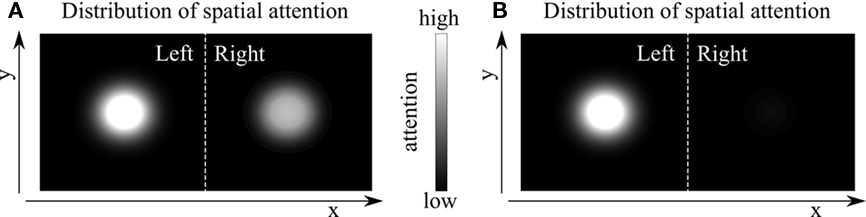
Figure 1. Alternative distributions of spatial attention in the two hemifields. By task design, the measure of attention in Cohen and Maunsell (2010) is intrinsically relative (e.g., more attention to the left versus right hemifield). Thus, it is not possible to distinguish whether attention within the source areas is split across hemifields (A), or instead there is a single spotlight of attention (B).
As noted by the authors, the proposal of two independent sources of attention, one per hemifield, is consistent with the psychophysical observation that twice as many stimuli can be tracked when they cover the two hemifields compared to when they are presented in the same hemifield (Alvarez and Cavanagh, 2005). However, whether there is complete hemifield independence, or whether the effect is gradual and can be described as bilateral advantage, is still under strong debate. In fact, more recent results show a trend in favor of the bilateral advantage (Chakravarthi and Cavanagh, 2009). Furthermore, the bilateral advantage scenario seems to be more consistent with the results in Cohen and Maunsell (2010). The correlation between attention and performance is slightly improved when V4 neurons in the opposite hemisphere are included in the analysis. Moreover, why would not the brain allocate the maximum amount of attention to both hemifields if they are independent? This would allow maximum performance independent of the change stimulus location.
Is a single source of attention compatible with the bilateral advantage? As pointed out in Chakravarthi and Cavanagh (2009), the bilateral advantage might be due to suppressive surrounds around the attentional focus in visual sensory areas. This suppressive effect may operate locally through the recruitment of inhibitory circuits, which does not affect areas in the opposite hemisphere. Thus, local surround suppression in visual sensory areas could underlie the increased difficulty in distributing attention within a hemifield versus between hemifields.
The question of whether attention operates as a single spotlight has long been a topic of research at the interface of systems neuroscience and cognitive psychology. By introducing new alternative scenarios for the neural circuits serving as the source of attention, this commentary stresses the necessity of further neurophysiological investigation. It remains undetermined which brain areas underlaid these attentional fluctuations in area V4. There is growing evidence showing that regions in prefrontal and parietal cortex have an important role as the source of attention control (Corbetta and Shulman, 2002; Gregoriou et al., 2009). Future experiments should use simultaneous multi-electrode recordings in prefrontal regions (e.g., frontal eye field) and a connected sensory region (e.g., V4) to explore the dynamics of the attentional employment and its correlation with behavior. Simultaneous recording in both hemispheres of the source region will provide insight into the degree of independence of the sources of spatial attention. Manipulating the separation of stimuli near the hemifield boundary could also probe the attentional source circuits, as interneuronal correlations between V4 hemispheres at small stimulus separation could reflect interactions within the attentional circuit and the spread of spatial attention across the hemifield boundary. Finally, having access to absolute measures of attentional modulation would also help to discard one of the alternative scenarios (Figure 1). For this, it would be necessary to interleave a third attentional condition in the task design that would not modulate the activity of the recorded V4 neurons, e.g., attention to a different stimulus feature in a location equidistant and far apart from the other two. More generally, the authors’ procedure is applicable to measure, at the circuit level and in a dynamical manner, inter-areal interactions that underlie other cognitive functions.
Acknowledgments
John D. Murray is supported in part by National Institutes of Health grant T15 LM07056 from the National Library of Medicine. Salva Ardid is supported by the Swartz Foundation. Authors thank Xiao-Jing Wang and Tatiana Engel for comments on a previous version of the manuscript.
References
Alvarez, G. A., and Cavanagh, P. (2005). Independent resources for attentional tracking in the left and right visual hemifields. Psychol. Sci. 16, 637–643.
Chakravarthi, R., and Cavanagh, P. (2009). Bilateral field advantage in visual crowding. Vision Res. 49, 1638–1646.
Cohen, M. R., and Maunsell, J. H. R. (2010). A neuronal population measure of attention predicts behavioral performance on individual trials. J. Neurosci. 30, 15241–15253.
Corbetta, M., and Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215.
Gregoriou, G. G., Gotts, S. J., Zhou, H., and Desimone, R. (2009). High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324, 1207–1210.
Reynolds, J. H., and Chelazzi, L. (2004). Attentional modulation of visual processing. Annu. Rev. Neurosci. 27, 611–647.
Citation: Murray JD and Ardid S (2011) What can tracking fluctuations in dozens of sensory neurons tell about selective attention?. Front. Syst. Neurosci. 5:35. doi: 10.3389/fnsys.2011.00035
Received: 26 February 2011;
Accepted: 17 May 2011;
Published online: 27 May 2011.
Copyright: © 2011 Murray and Ardid. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: salva.ardid@yale.edu