Conducting Polymers (CPs) are attractive in the field of bioelectronics for their excellent biocompatibility and unique electrical and chemical properties. Hence, CPs, such as, polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), and polyanilline (PANI), have been extensively studied for their potential applictions in biosensing, implantable electrodes, tissue engineering, and actuators. Futhermore, when patterned, CP films, especially with various surface chemistries, provide versatile and sophisticated building-blocks for bioelectronics[1]. In this context, we recently invented a novel and smart technique of hydrogel-mediated electrodeposition to directly pattern CP films with spatially-addressable chemistries[2]. This unique technique employs a topographically patterned agarose gel to deliver polymer precursors to the working electrode during the CP electropolymerization. Hydrophilic agarose hydrogel offers an ideal storage space not only for the polymer precursors but also for other fragile biomolecules to be incorporated into the CP network. Herein, we extend this technique to create CP films with molecular gradients and subjected the surface for the cell adhesion study to demonstrate its potential applications in cellular engineering.
In order to create molecular gradients on CP films, we exploited asymmetric distribution of dopant molecules in agarose hydrogel. Briefly, we first loaded pyrrole monomer onto a topographically patterned agarose gel and then exposed this hydrogel to two dopant solutions, polystyrene sulfonate (PSS) and 10-mer laminin peptide, at the two opposite ends of the hydrogel, allowing the two dopants to diffuse through the hydrogel network. Then, we subjected the inked hydrogel for the hydrogel-mediated electropolymerization as previously described[2]. The resulting CP substrate had a continuous PPy film deposited with no apparent deviation. Further analysis of the film surface using Fourier-transform infrared (FT-IR) spectroscopy revealed that there was uneven distribution of dopant molecules along the PPy film. This patterned PPy film with PSS and laminin peptide gradient was then applied for cell adhesion study. Here, human glioblastoma (U87) cells were cultured on sterilized PPy substrates for 48 hours, and the number of live cells along the PPy line was counted. Surprisingly, U87 showed biased attachment and growth on the laminin peptide-concentrated portions (Figure 1), indicating a potential application of this technique to provide an effective substrate to control cellular behavior.
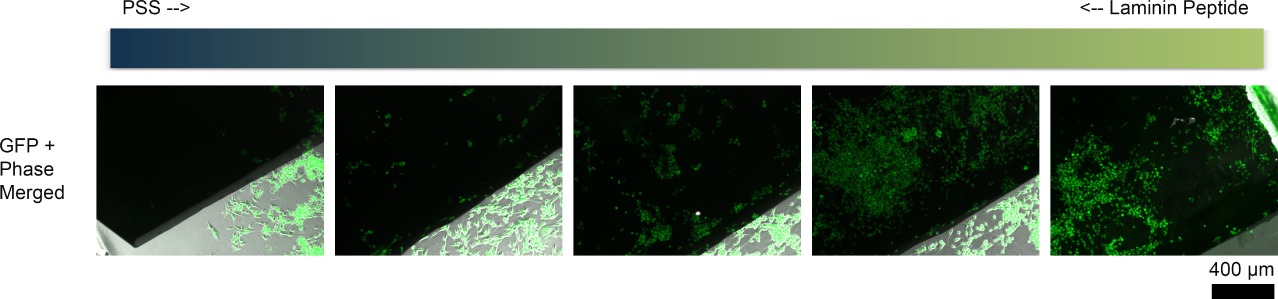
Figure 1. The images of U87 cells (phase + green (indicating live cells) on PPy films with gradients of PSS and laminin peptide.
In conclusion, we present a simple and multifaceted strategy to micropattern CP films with high fidelity and spatially addressable surface chemistries. Most importantly, this unique approach enables efficient fabrication of fixed molecular gradients on surfaces. Fine optimization of the technique is anticipated to broaden its applications in future organic bioelectronics.
References:
[1] Park S, Kang YJ, Majd S. Adv. Mater. In Press
[2] Park S, Guang Y, Madduri M, Abidian MR, Majd S. Adv. Mater. 2014. 26:18, 2782-2787.