Introduction: Sulfonic glycosaminoglycan (SGAG) are one of the major component of the extracellular matrix. They play an important role in the proliferation and the differentiation of cells. In this study, we synthetized an artificial SGAG by modifying chitosan with 4-formyl-1,3- benzene disulfonic acid (BZ2S) to yield in the presence of sodium cyanoborohydride a disulfonated chitosan derivatives (CHT2S). The obtained polymer was characterized by FTIR, H1NMR, and by elemental analysis. Subsequently, based CHT2S electrospun scaffolds were produced.
Experimental Methods: Synthesis of CHT2S: CHT (5g) was dissolved in 500 ml of 0.5% of aqueous solution of acetic acid .Methanol (650mL) was added to the chitosan solution. Sodium cyanoborohydride (NaBH3CN) (3g) was dissolved in the solution under vigorous stirring and after 3 min BZ2S dissolved in 150ml of water was added. The resulting precipitate was filtered and washed with water and dried, yielding a powder[1]. Different CHT2S were obtained by varying the ratio (n) between SO3-/NH3+ (n= 2.0 ,1.0, 0.5).
Characterizations: The chemical composition and the quantification of the degree of substitution of the glucosamine units of CHT were evaluated by FTIR and 1H NMR 300Mhz. The percentage of sulfur was measured using elemental analysis. The morphology of the chrome sprayed nanofibres was observed with a scanning electronic microscopy (Hitachi S-4700 SEM) operating with an acceleration voltage of 5 kV.
Electrospinning: CHT2S (n=2.0, 1.0, 0.5)/PEO mixture (ratio 9/1) were dissolved in NaOH (0.1M). The applied voltage was at 15kV; The flow rate was fixed at 0.5mL/h and the tip-to-collector distance at 20cm. The obtained nanofibres were crosslinked by genipin (0.1wt%).
Results and Discussion: The CHT2S structure was confirmed by FTIR thanks to the new bands relative to sulfonic acid hydrates at 2400-2000 cm-1 and 1230-1235 cm-1, and to substituted benzene groups below 800 cm-1. NMR spectrum evidenced the presence of the grafted aromatic substituent through peaks appearing above 7ppm. The DS were calculated by nmr and elemental analysis. The results are shown in the following table:
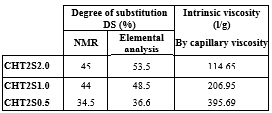
DS= % of NH2 substituted by benzenesulfonate groups

Figure 1: SEM images of nanofibres obtained with different CHT-2S2.0/PEO concentrations 5wt% (left), 6wt%(middle),7wt% (right)
Figure 1, shows that the concentration plays an important role in the yielding of beads-free nanofibres

Figure 2: SEM images of nanofibres obtained from: CHT2S0.5 (left) CHT2S1 (middle) CHT2S2 (right)
Furthermore, it was observed that by increasing the ratio SO3-/NH3+ from 0.5 to 2, defect-free nanofibres were obtained (figure 2). It could be explain by the increasing of the DS (see table), as the more the chitosan is substituted the lower viscosity we obtain. Genipin crosslinking improved the stability of the electrospun mats as the nanofibrous structure resisted to a prolonged stay in water and in PBS buffer (pH7.4). Cytocompatibility study and cell proliferation tests with fibroblasts NIH3T3 are now in progress in order to evaluate the biological properties of our obtained membranes and to inform us if our CHT2S nanofibres meet the characteristics of GAGs.
Conclusion:
Stable and defect-free nanofibres were obtained by electrospinning of disulfonated chitosan with different DS. The obtained membranes are a suitable candidate for tissue engineering application.
This work was supported by Region Nord-Pas-de-Calais, University of Lille and Federation Cheuvreul
References:
[1] Martel et al, J Appl Polym Sci, 59, 647-654 (1996)