Introduction: Aeration of a fibrin sealant using a patented mixing device leads to the generation of a novel biopolymer – fibrin foam[1],[2]. The foam sets quickly and can be applied to vertical and inverted surfaces. This research evaluates the effects of aeration on the physical and biological characteristics of the foam and its biocompatibility.
Materials and Methods: Generation of fibrin foam: The foam was prepared using a dual syringe mixing device – one containing thrombin solution plus air and the other filled with a fibrinogen solution from a commercially available fibrin sealant. The constituents were repeatedly passed through a patented, sintered porous polyethylene disc (Baxter Healthcare Corporation) for aeration.
Preparation and structural characterization: Foam preparations were altered by the number of passes through the mixing disc and variation of constituent concentrations and volumes. Porosity and fibrin clot structure were examined within various foams via scanning electron microscopy. Pore sizes were measured by Feret’s diameter using FIJI ImageJ.
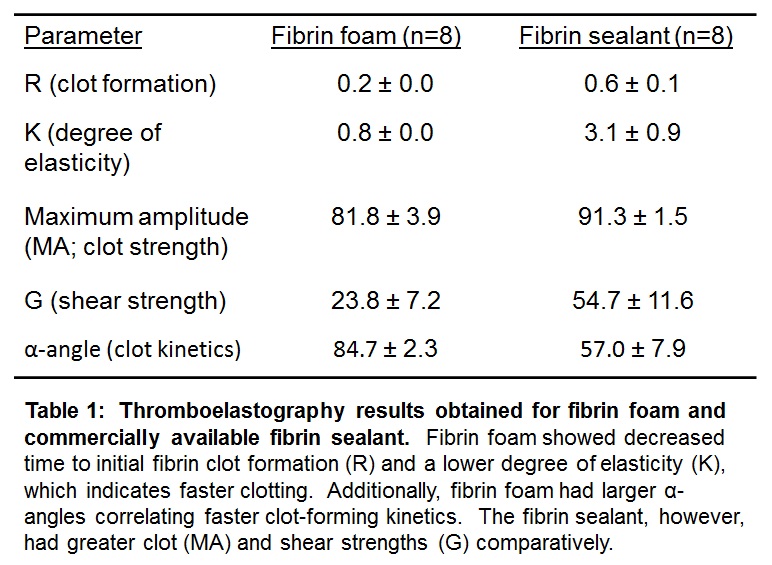
Biological and biomechanical testing: Thromboelastography (TEG 5000) was used to analyze clot strength and formation kinetics of fibrin foam compared to a commercially available fibrin sealant. A pull test (MTS Insight) was applied to fibrin foam to measure tensile strength.
Biocompatibility: Normal human dermal fibroblasts, normal human epidermal keratinocytes, and human umbilical vein endothelial cells (PromoCell) were seeded on fibrin foam clots. Cells were incubated on the clots for 2, 24, and 48 hour time points. Supernatants were analyzed for lactate dehydrogenase using CytoTox96 Assay (ProMega).
Results and Discussion: A porous foam was generated by aeration, and fibrin foam maintained fibrin clot structure surrounding the pores when compared to the fibrin sealant.
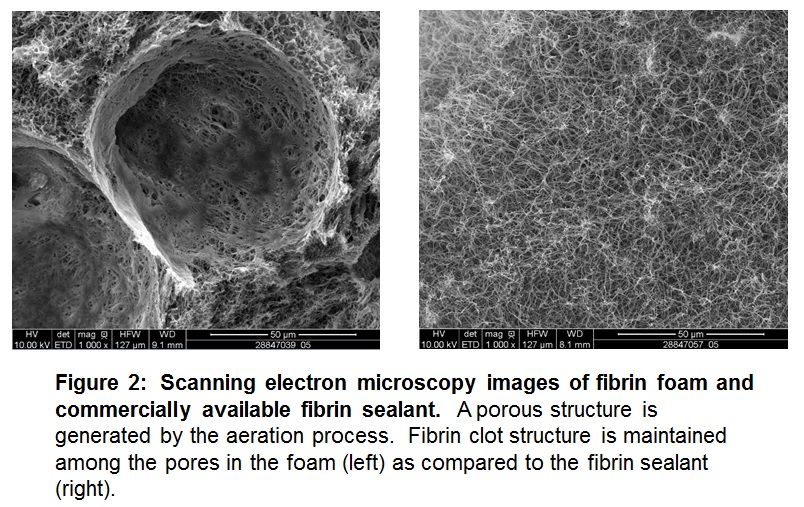
The mean pore size distribution in the foam was unchanged by manipulating the number of passes through the mixing disc and varying the constituent concentrations. Resulting mean pore sizes were found to be ≤200µm, which is the maximum optimal pore size for the growth and viability of the cell types analyzed[3]-[5]. Cell viability remained consistent across the controls and foams evaluated. TEG analysis showed a slight decrease in mechanical strength of the foam compared to the sealant; however, fibrin foam had substantially faster clotting time and initial clot formation.
Tensile strength of the foam decreased compared to the sealant.
Conclusion: This work has detailed initial characterization of a novel biopolymer, fibrin foam. Fibrin foam has distinct gross and minute characteristics, including cellular biocompatibility and an open-pore structure, which may allow for cell migration and growth. Combined with the biodegradable and antibacterial nature of fibrin sealants, these attributes make fibrin foam a potential candidate to improve wound healing.
James DiOrio; Jason Mantei; Karen Applehoff; Peter Park; Kevin Lewis
References:
[1] U.S. Patent #8,512,740 B2. Aug 20, 2013. Fibrin Foam and Process for Making. Delmotte, Y.A.
[2] U.S. Patent #8,753,670 B2. Jun 17, 2014. Fibrin Foam and Process. Delmotte, Y.A
[3] O’Brien, F., Harley, B., Yannas, I. et al. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 26 (2005): 433-441.
[4] Dhandayuthapani, B., Yoshida, Y., Maekawa, T. et al. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int Journal of Polymer Science (2011): 1-19.
[5] Chang, H. and Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. Regenerative Med Tissue Eng (2011). ISBN: 978-953-307-663-8.