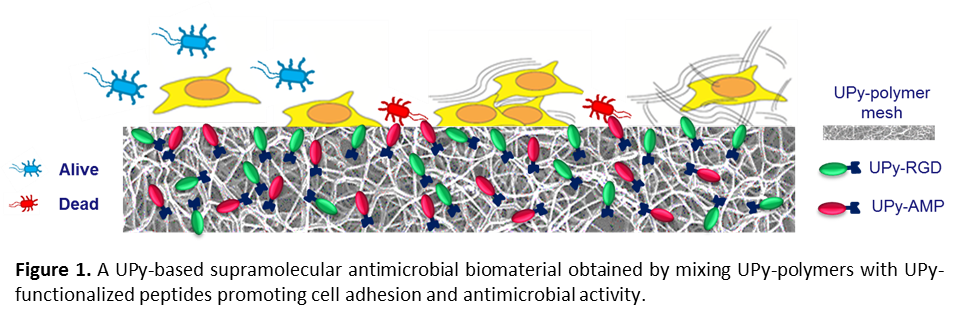
Introduction: Preventing biomaterial associated infections represents one of the main challenges for a new generation of biomaterials, which can significantly improve the quality of life. One strategy to achieve this goal is the coating of medical devices and implants with antimicrobial agents. Among these, Antimicrobial Peptides (AMPs) are currently the most promising, mainly because of their broad spectrum of action and low risk to promote bacterial resistance[1]. In this context, our research aims to establish a new methodology for the incorporation of AMPs into biodegradable implantable polymers based on supramolecular interactions. A modular approach based on supramolecular building blocks enables to easily prepare instructive, biodegradable scaffolds by simply mixing-and-matching of short prepolymers and peptides functionalized with hydrogen-bonding ureido-pyrimidinone (UPy) moieties[2]. In addition, different bioactives can easily be added, enabling to prepare multifunctional biomaterial and to fine tune them according to specific clinical needs. Indeed, tissue engineering and regenerative medicine often require tissue integration; this can be achieved by enhancing autologous cell adhesion on the biomaterial. Here we report on the development of a cell-adhesive antimicrobial supramolecular biomaterial with improved in-situ tissue regenerative capacity for cardiovascular, renal and urological applications, obtained by simply mixing UPy-polymers with various UPy-functionalized peptides promoting both eukaryotic cell adhesion (e.g. RGD)[3] and antimicrobial activity (Figure 1).
Materials and Methods: All the peptides were synthesized via Fmoc-based manual solid phase peptide synthesis (SPPS). A carboxylic acid UPy-synthon (UPy-COOH) was coupled to the N-terminus of the peptide using HATU. Purification of the UPy-peptides was carried out through preparative HPLC.
Results and Discussion: A small library of UPy-functionalized peptides is designed and synthesized. UPy-functionalized RGD peptides were easily prepared by coupling a UPy-COOH synthon to the protected peptides, while these were still anchored to the resin on which they were synthesized. Antimicrobial peptide sequences were chosen from literature[4]-[6]. Different conditions for Fmoc solid phase strategy have been explored to succeed in the synthesis of the AMPs. The best results in terms of yields and purity were obtained using Sieber amide resin, OxymaPure/DIPCDI as coupling agent and adding a surfactant (Triton-X) to improve the swelling of the resin. Attempts to couple the UPy-COOH synthon to AMPs using the same solid-phase procedure developed for the RGD peptides failed to give the desired products. An in solution strategy for coupling AMPs to the UPy-synthon was therefore developed: AMPs synthesized on Sieber resin were cleaved under mild acidic conditions and then coupled to the UPy-COOH synthon in solution using HATU as coupling agent.
Conclusions: A library of UPy-peptides with either cell-adhesive or antimicrobial properties was successfully prepared. These peptides are going to be used in combination with UPy-polymers to prepare supramolecular biomaterials able to induce tissue formation while preventing infections.
Acknowledgements: This research has received funding from the Union‘s Seventh Framework Programme (FP/2007-2013) under Grant Agreement number 310389 (BIP-UPy).
References:
[1] Salwiczek, M.; Qu, Y.; Gardiner, J.; Strugnell, R. A.; Lithgow, T.; McLean, K. M.; Thissen, H. (2014) Trends in Biotechnology, 32(2), 82–90.
[2] Dankers, P.Y.W.; Harmsen, M.C.; Brouwer, L.A.; van Luyn, M.J.A., Meijer, E.W. (2005) Nat Mat, 4(7), 568-574.
[3] Yang, F; Williams, C. G.; Wang, D. A.; Lee, H. ; Mason, P. N.; Elisseeff, J. (2005) Biomaterials, 26, 5991-5998.
[4] Avrahami, D.; Oren, Z.; Shai, Y. (2001) Biochemistry, 40, 12591–12603.
[5] Bagheri, M.; Beyermann, M.; Dathe M. (2011) Bioconjugate Chem., 23, 66-74.
[6] Yang, S.-T.; Shin, S. Y.; Lee, C. W.; Kim, Y.-C.; Hahm, K.-S.; Kim, J. II. (2003) FEBS Letters, 540(1-3), 229-233.