Introduction: Healthy teeth are attached to the jawbone through an exquisitely controlled mineralization process. In periodontitis this attachment is destroyed by bacterial invasion. In order to develop more effective strategies to regenerate the ligament-tooth attachment, it is critical to have a better understanding of the biomineralization of collagen fibrils in the formation of this hard-soft tissue interface. While non-collagenous proteins have been extensively investigated as promoters and inhibitors of collagen mineralization[1] the role of glycosaminoglycans (GAGs) is unclear, notwithstanding some suggestions that GAGs might maintain the ligament in its unmineralized state[2].
The aim of this study was to determine the role of GAGs in collagen mineralization at the periodontal ligament–cementum junction. We used our recently developed tissue based in vitro model, in which the extracellular matrix of demineralized mouse periodontal sections retains sufficient information to direct preferential remineralization of the natively mineralized tissues[3].
Materials and Methods: To determine the role of matrix GAGs in control of mineralization, demineralized mouse periodontal sections were exposed to chondroitinase ABC and hyaluronidase, which digest chondroitin sulfates, and hyaluronan respectively. The GAG removal was quantified using a modified DMMB assay, and polyacrylamide gel electrophoresis (PAGE). Gels were stained with Stains-all method, followed by silver staining to maximize detection. Tissue sections were remineralized as described previously[3] and examined by TEM. Sections digested (D) with chondroitinase ABC and hyaluronidase were compared to the control untreated sections (C).
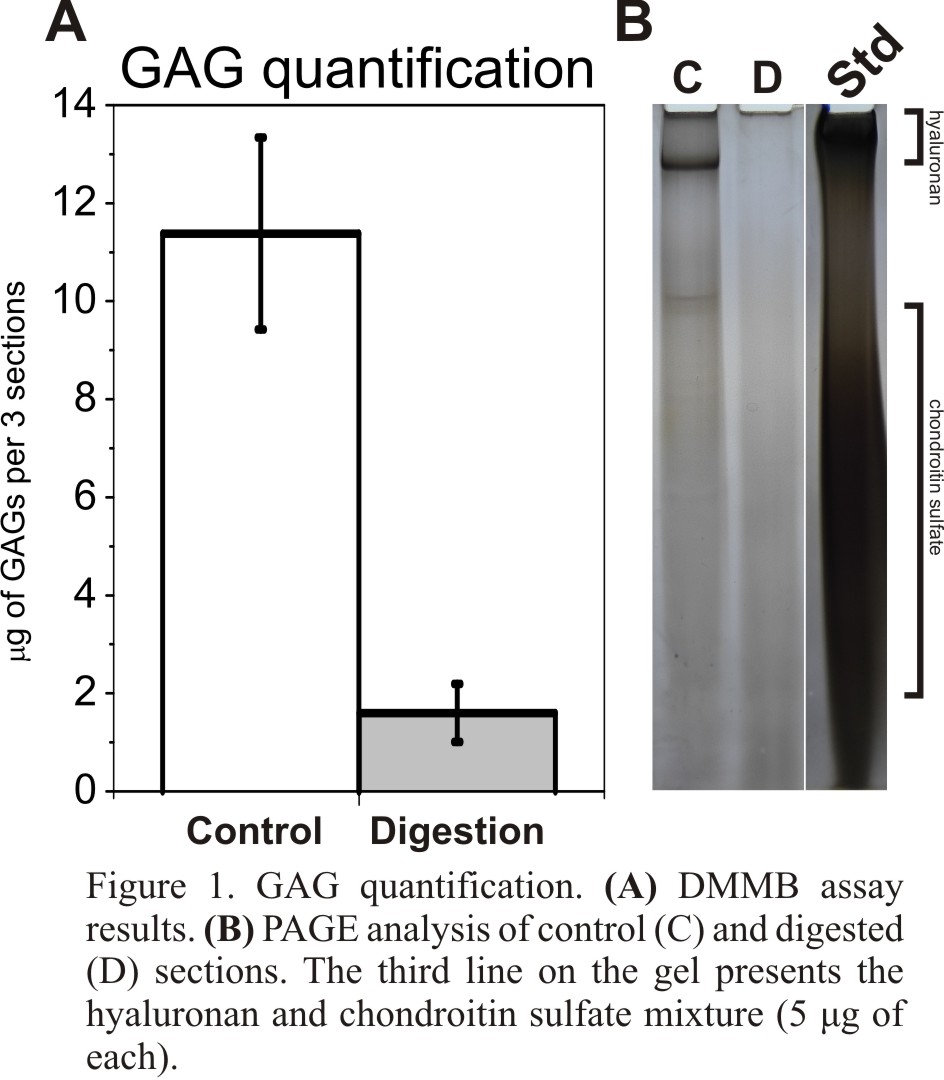
Results and Discussion: Our analysis shows significant removal of GAGs from tissue sections following enzymatic digestion with chondroitinase ABC and hyaluronidase. A DMMB assay, which specifically detects sulfated GAGs, showed an 80% reduction of GAGs compared to control sections (Fig. 1A). PAGE analysis confirmed DMMB results. There were bands observed in control samples, which were not detected in digested samples (Fig. 1B).
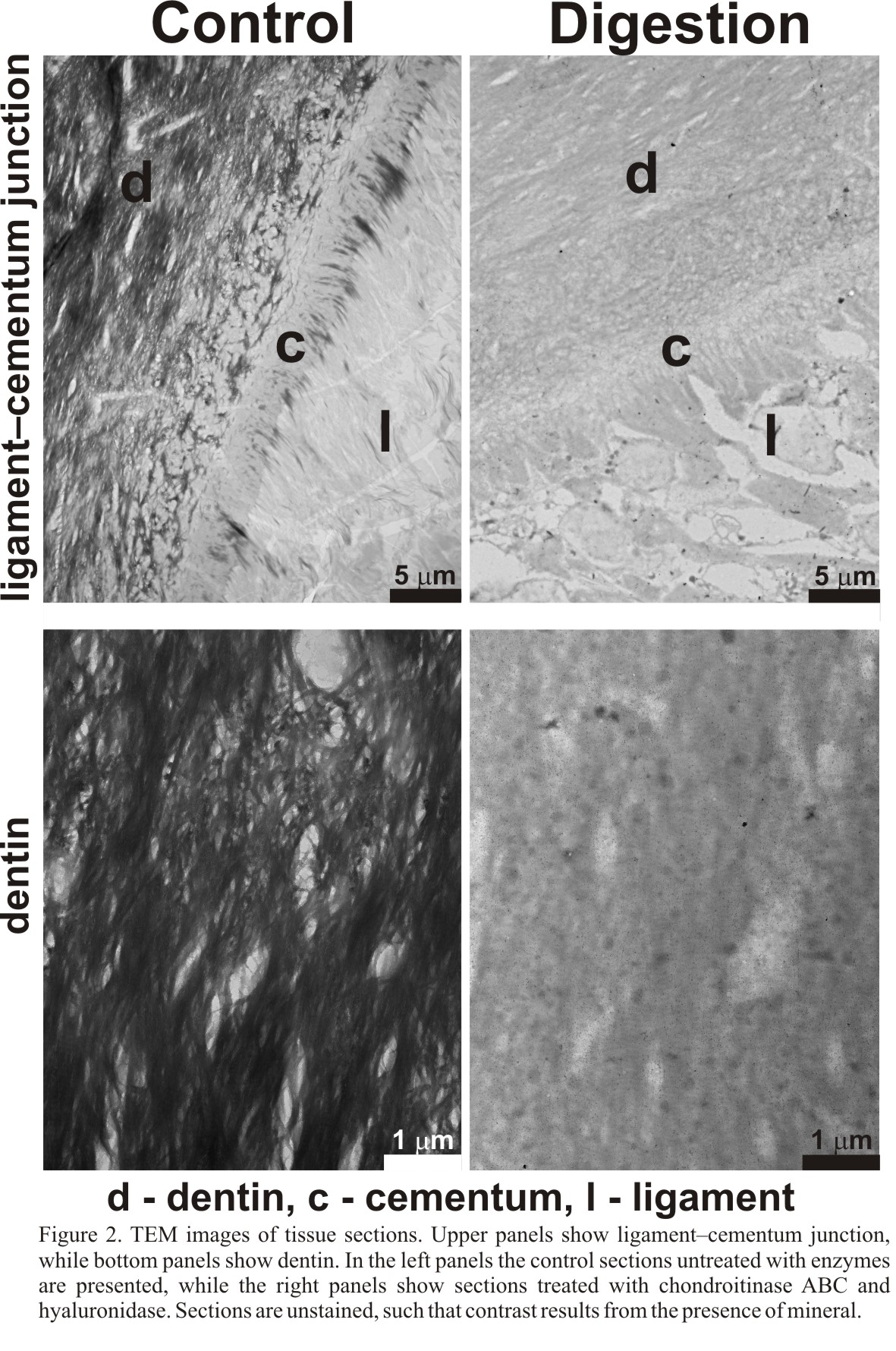
TEM analysis of remineralized tissue sections has shown that GAG removal reduced the rate of remineralization compared to the control (Fig. 2). The selectivity of remineralization was maintained in all cases; only dentin and cementum remineralized, while the ligament remained unmineralized. These results differ from previous findings where GAG removal caused ligament mineralization in sheep teeth[2]. The acidic character of GAGs implies possible roles in calcium ions binding, nucleation and control of crystal growth. Our preliminary results suggest that GAGs, along with acidic phosphoproteins, are responsible for promoting mineralization in dentin/cementum rather than inhibiting crystal formation in ligament.
Conclusion: Our findings indicate that GAGs might play a role in control of nucleation and/or crystal growth during collagen mineralization.
This study was supported by the Canadian Institutes of Health Research.; Project supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014-2018
References:
[1] A. George and A. Veis, “Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition,” Chem. Rev., vol. 108, no. 11, pp. 4670–4693, 2008.
[2] J. Kirkham, S. J. Brookes, R. C. Shore, W. A. Bonass, and C. Robinson, “The effect of glycosylaminoglycans on the mineralization of sheep periodontal ligament in vitro,” Connect. Tissue Res., vol. 33, pp. 23–29, 1995.
[3] A. J. Lausch, B. D. Quan, J. W. Miklas, and E. D. Sone, “Extracellular Matrix Control of Collagen Mineralization In Vitro,” Adv. Funct. Mater., vol. 23, no. 39, pp. 4906–4912, Oct. 2013.