Introduction: In recent years, a shift has been observed from the use of simple single-layered scaffolds for tissue engineering and drug delivery, to a combination of various materials and multilayered techniques to achieve complex three-dimensional (3D) scaffolds.[1] Hydrogels are promising scaffolds for tissue engineering due to their high water content, biocompatibility and tissue-like physical and mechanical properties.[2] Herein, we characterize a bi-layer hydrogel system formed from photoclickable PEG hydrogels using thiol-nobornene macromers. This polymerization is promising for tissue engineering because of the orthogonal nature of network formation and the rapid gelation afforded by this chemistry.[3] This work, thus investigates the interface that forms between two adjacent layers and the factors that influence the local and overall bulk properties of bi-layer hydrogels by characterizing (a) the interface thickness and the effects of diffusion, (b) the mechanics of multi-layer hydrogels, and (c) the localized strains experienced within each layer of a bilayer hydrogel.
Methods: A macromer solution consisting of either 10, 15, or 25% (w/w) 8-arm PEG-norbornene (20,000 molecular weight (MW); JenKem Technology USA Inc.), PEG-dithiol (1000 or 3400 MW; Sigma-Aldrich) crosslinker at 1:1 thiol:ene ratio, and 0.05% (w/w) photoinitiator, 1-(4-(2-Hydroxyethoxy)-phenyl)-2-hydroxy-2-methyl-1-propane-1-one (I2959; BASF), in diH2O was photopolymerized via ultraviolet light (10 minutes, 5-10 mW/cm2, 352 nm). Hydrogels were formed in cylindrical molds and following polymerization of the first layer, the second layer was deposited and then polymerized after prescribed times, referred to diffusion time (td). Thiolated fluorophores were included to visualize the interface. Compressive testing was conducted on a Mechanical Testing System (MTS). Equilibrium swollen hydrogels (n = 3/group) were tested in unconfined compression at a constant strain rate of 0.5 mm/min to 50% strain (2 mN pre-load; limited by a 9 N max load with a total load cell capacity of 10 N).
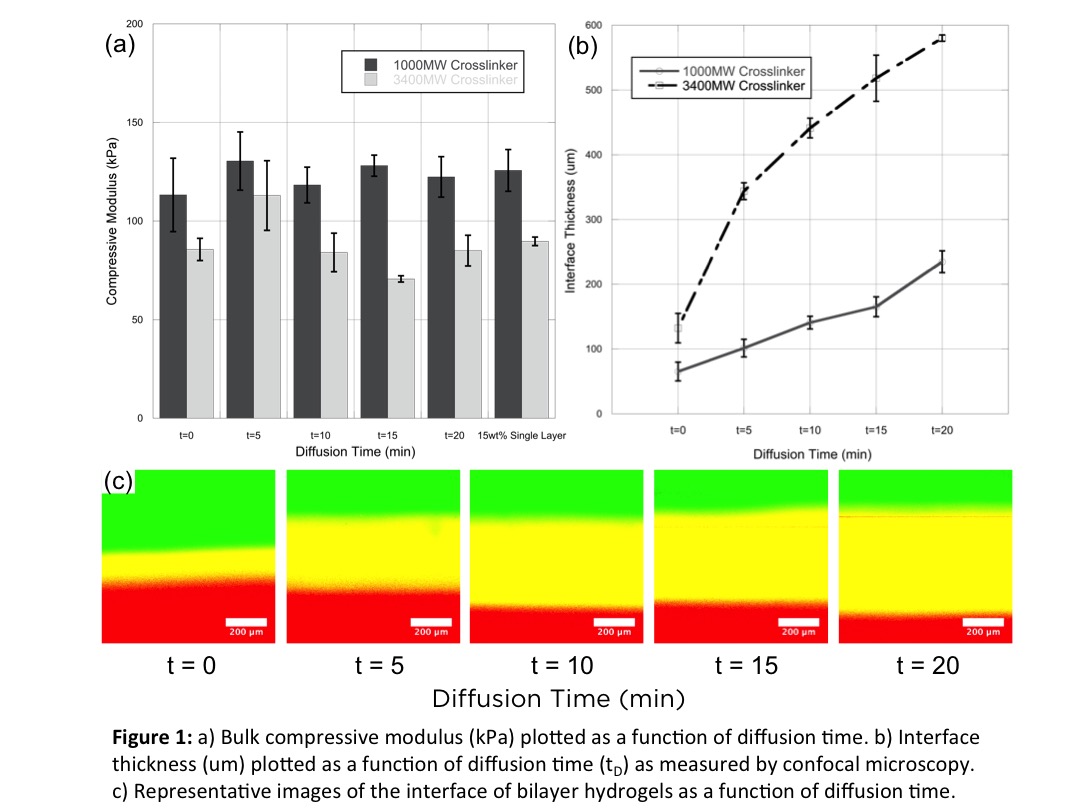
Results: Hydrogels developed from this method were tested first for their mechanics. For the diffusion times explored, hydrogels showed mechanical properties that were consistent to their single layer counterparts (Fig. 1a). At up to 50% strains, the hydrogel interfaces experienced no failure and remained intact throughout testing. We believe that this robustness is due to the orthogonal nature of network formation, resulting in free reactive groups in the first layer that allows for covalent coupling between successive layers. The length scales of hydrogel interfaces were then examined by confocal microscopy. Here, the diffusion time had a direct effect on the size of the interface (Fig. 2c). As the diffusion time increased the interface, depicted as the overlap of red and green fluorescence, increased approximately linearly (Fig. 2b). In addition, a larger crosslinker, which in turn results in a larger mesh size and greater diffusivity, resulted in even larger interfaces.
To characterize the mechanical properties across the interface, atomic force microscopy (AFM) was utilized. From AFM, it was determined that the hydrogels mirror mechanics obtained from bulk compressive tests. The interface thickness is also similar to that measured by confocal microscopy. And more importantly, that there is a linear gradient in mechanics across this interface, plateauing at the bulk modulus of the material (Fig. 2c).
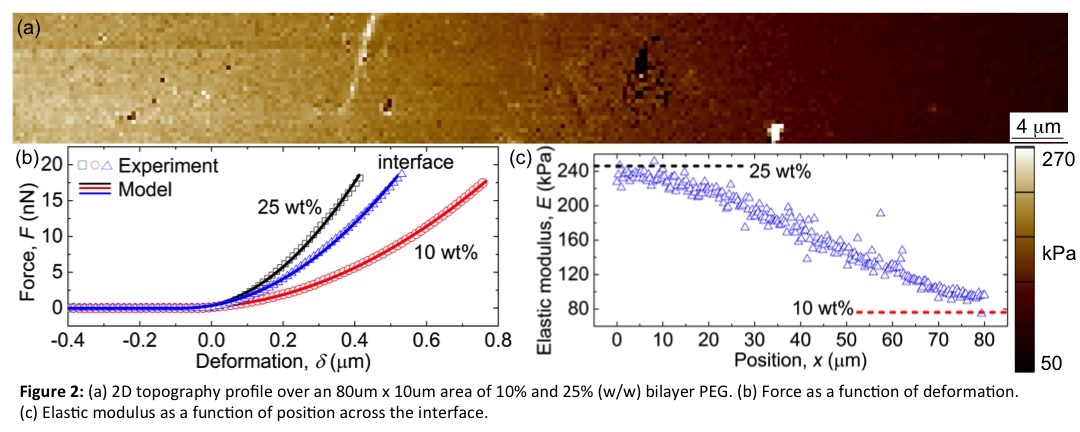
Conclusions: Photoclickable PEG thiol-ene hydrogels were employed to form multi-layered 3D scaffolds. From these initial results, interfaces can be adjusted from 50-500 um, while still remaining robust. From AFM, it was determined that interface mechanics change gradually across the length of the interface, which may further contribute to the robustness of the material.
NIH Pharmaceutical Biotechnology Training Grant; NSF Career Award (0847390)
References:
[1] Verhulsel, M., Vignes, M., Descroix, S., Malaquin, L., Vignjevic, D. M., & Viovy, J. L. (2014). A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials, 35(6), 1816–1832. http://doi.org/10.1016/j.biomaterials.2013.11.021
[2] Tibbitt, M. W., & Anseth, K. S. (2009). Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and Bioengineering, 103(4), 655–663. http://doi.org/10.1002/bit.22361
[3] Hoyle, C. E., & Bowman, C. N. (2010). Thiol-ene click chemistry. Angewandte Chemie (International Ed. in English), 49(9), 1540–73. http://doi.org/10.1002/anie.200903924