Purpose: A study was conducted in order to determine whether or not hydroxyapatite would form on a bone void filler containing bioactive glass. Hydroxyapatite (HAp) forms the predominant mineral component of vertebrate bone and tooth enamel[1]. Bioactive glass interacts with ions in the body to form HAp layers on the surface of the glass; the similarity of this surface to naturally occurring hydroxyapatite provides optimal conditions for new bone formation[2]. In this study, a bone void filler containing bioactive glass was immersed in simulated body fluid (SBF)[3][4][5] for a period of 24 hours to initiate hydroxyapatite formation. Vioxx and Mo-Sci bioactive glass were used as positive controls since various Vioxx size distributions are present in the bone void filler and Mo-Sci is competitor material. Stainless steel and borosilicate spheres (McMaster Carr) were used as negative controls since these samples had spherical morphology similar to the bioactive glasses with the exception of having smooth surfaces and borosilicate is similar to bioatcive glass in terms of material composition. Fourier transform infrared spectroscopy (FTIR) and scanning electron microscope (SEM) micrographs were used to detect HAp presence; characteristic FTIR bands for HAp are seen between 560 and 610 cm-1[1] and SEM micrographs allowed for qualitative assessment of crystalline morphology. FTIR spectra and SEM micrographs indicated HAp formation for the bioactive glass-containing bone void filler.
Methods: Each sample was immersed in SBF at 37⁰C at 175 RPM for 24 hours to propagate HAp formation. Following immersion, samples were dried with acetone to halt surface reactions and prevent additional HAp formation. FTIR spectra were obtained using the Nicolet iS10 FTIR with Smart Orbit ATR and diffuse reflectance stage; spectra were collected from 400cm-1 to 2000cm-1 with 64 scans and automatic background subtraction. The SEM Hitachi SU5000 (University of Florida, Gainesville, FL) was implemented for obtaining SEM micrographs at magnifications ranging from 3 to 500µm.
Results: A sample size of n=3 was assessed for FTIR. A sample size of n=1 or 2 was assessed for SEM; multiple micrographs were taken at various magnifications and negative controls were only assessed following immersion. Figure 1 displays the FTIR for one positive control (Vioxx, 90-710µm particle size), one negative control (borosilicate spheres, 0.094 in diameter), and the bone void filler test sample; both bone putty samples displayed were immersed in SBF while the two control materials are compared before and after immersion. The FTIR spectra show HAp formation for the Vioxx glass and the bone void fillers following immersion, as indicated by spectral peaks around 600 cm-1. Figure 2 displays the SEM micrographs for the Vioxx glass (90-710µm particle size) before and after immersion and the stainless steel, borosilicate, and two bone void filler samples following immersion; micrographs for negative controls prior to immersion were not captured. The SEM micrographs display HAp formation for the Vioxx glass and bone void filler samples following SBF immersion; HAp is not present on Vioxx prior to SBF immersion or on the borosilicate and stainless steel samples following immersion.
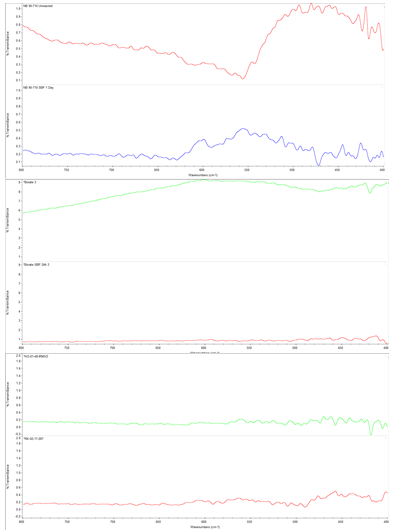
Figure 1: From top to bottom: Plots 1 and 2 are Vioxx glass prior to and following immersion, 3 and 4 are borosilicate prior to and following immersion, and 5 and 6 are two bone void fillers following immersion

Figure 2: The top two SEM micrographs represent Vioxx prior to (left) and following immersion (right); the middle images represent stainless steel (left) and borosilicate following immersion; the bottom images represent the two bone void filler samples following immersion
Conclusions: Immersing a bone void filler containing bioactive glass in SBF resulted in HAp formation as detected using FTIR and SEM; the positive control materials exhibited HAp formation and the negative controls did not express HAp, as expected. The formation of HAp indicates that the bone void filler is a bioactive device. A next step in the study will be to further investigate HAp presence using qualitative X-ray Powder Diffraction (XRD).
References:
[1] Stanciu, G. A., et al. "Investigation of the hydroxyapatite growth on bioactive glass surface." Journal of Biomedical & Pharmaceutical Engineering 1.1 (2007): 34-39
[2] Hench, L. "Bioactive Materials: The Potential for Tissue Regeneration." J. Biomed. Mat. Res.41: 511-518 (1998)
[3] T. Kokubo, H. Kushitani, S. Sakka, T. Kitsugi and T. Yamamuro, "Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W", J. Biomed. Mater. Res., 24, 721-734 (1990)
[4] ISO 23317: Implant for surgery-In vitro evaluation for apatite-forming ability of implant materials. 1-10-2012
[5] Petrochenko et al., “Development of Standardized Test Method for Evaluation of Nano-Crystalline Hydroxyapatite Forming Ability of Orthopedic, Dental and Craniofacial Device Materials,” FDA Center for Devices and Radiological Health