Introduction: Self-assembled micellar structures have been extensively studied as drug carriers for cancer and other diseases. These micelles, which are generally formulated with amphiphilic block copolymer, allow controlled release of encapsulated drugs[1]. Poly(β-amino ester) (PBAE) is a class of biodegradable polymers that is widely investigated as non-viral vectors for gene and drug delivery[2]. Neutrally charged, hydrophilic poly(ethylene glycol) (PEG) molecules can be coated onto a positive surface of nanoparticles to prevent aggregation and clearance from non-specific adsorption of proteins on the surface[3]. In this study, PEG was end-capped to hydrophobic PBAE polymer to provide amphiphilicity, trigger micellar self-assembly in aqueous solution, and promoted particle stability.
Materials and Methods: To synthesize PEG-PBAE, acrylate-terminated hydrophobic PBAE was reacted with PEG molecules to produce PEG-PBAE-PEG amphiphilic copolymers. Gel permeation chromatography and 1H NMR analysis verified successful PEG end-capping. After micellar self-assembly in aqueous solution, the micelles were characterized by measuring the surface charge by dynamic light scattering, size by nanoparticle tracking analysis, and critical micelle concentration (CMC) by pyrene fluorescence. Micellar morphology and aggregation were visualized by TEM. The cellular uptake and cytotoxicity of doxorubicin (dox)-loaded micelles and blank micelles were evaluated in HUH7 human hepatocellular carcinoma cells using comparable free dox concentration for comparison.
Results: PEG-PBAE copolymer was determined to have a CMC of .0155mg/mL. Nanosight data suggests micelle stability due to consistent size in water, PBS, 25%, 50%, and 75% v/v FBS-PBS over 24 h with hydrodynamic diameter of approximately 220 nm. Zeta potential of blank micelles was near neutral at -1.88 mV. Loading efficiency of doxorubicin in PEG-PBAE micelles was determined to be 2.1% by mass. Micelles exhibited 80% cellular uptake in Huh7 cells. Approximately 75% of the cells were killed when exposed to dox-loaded micelles or equivalent concentration of free dox.
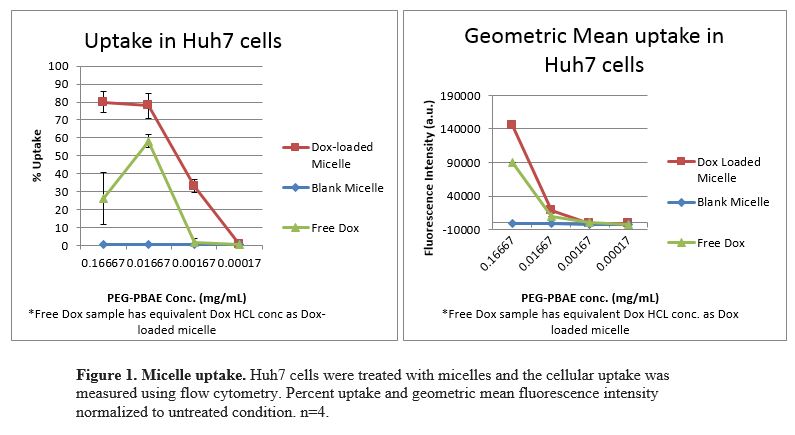
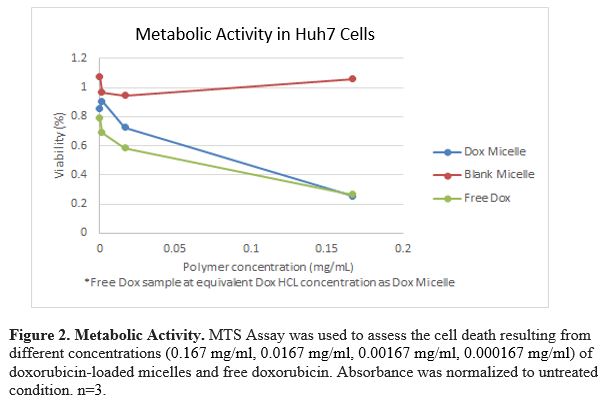
Conclusions: These results indicate that doxorubicin-loaded micelles can be formulated with a novel PEG-PBAE copolymer. PEG-PBAE micelles are stable over time and can successfully deliver drug cargo inside cancer cells and cause mortality
The authors acknowledge Samsung Scholarship and NIH 1R01EB016721 for funding, Johns Hopkins Microscope Facility core for TEM, and Wilmer Core Grant (P30-EY001865, Imaging and Microscopy Core Module) for flow cytometry.
References:
[1] Lasic, D.D, Nature, 1996, 380, 561
[2] Green, J.J., et al. Acc Chem Res, 2008, 41(6): 749-759.
[3] Kwon, G.S., et al. Adv Drug Deliv Rev, 2002, 54:169-190