Introduction: A great deal of research effort has been directed to engineer the porosity characteristics and drug loading techniques on bioceramics in order to control binding and release kinetics[1][2][3][4]. The purpose of the present study is to understand the role of ceramic functional groups on drug binding and release kinetics. The surface chemistry of alpha-cristobalite (Cris) was modified via doping with phosphorous. The silicate-phosphate ionic substitution was analyzed and correlated to surface chemistry, drug adsorption and release kinetics.
Materials and Methods: Cris particles (90-150um) were doped with 0% (P0), 0.0625% (P.0625), 0.125% (P.125), 0.25% (P.25) or 0.5% (P.5) mole % of P using orthophosphoric acid and thermal treatment. The material structure was analyzed using XRD with Reitveld Refinement and FTIR with Gaussian fitting. Cris samples were immersed separately in DI water for 2 days and the concentrations of dissolved Si and P ions were measured using ICP-OES. Samples before and after immersion were loaded with vancomycin and the release kinetics was measured in PBS. Statistical analysis was completed using the student’s t-test, (n=5, p < 0.05).
Results: XRD results showed an increase in the size of the unit cell of Cris as P content increased. Releasing the P from the surface increased the population of the O-Si-O functional groups (Fig. 1) and enhanced drug adsorption. Release kinetics study showed a decrease in the rate of drug release as the P content increased (Fig. 2). After P release from the material surface the rate of drug release was significnatly enhanced.
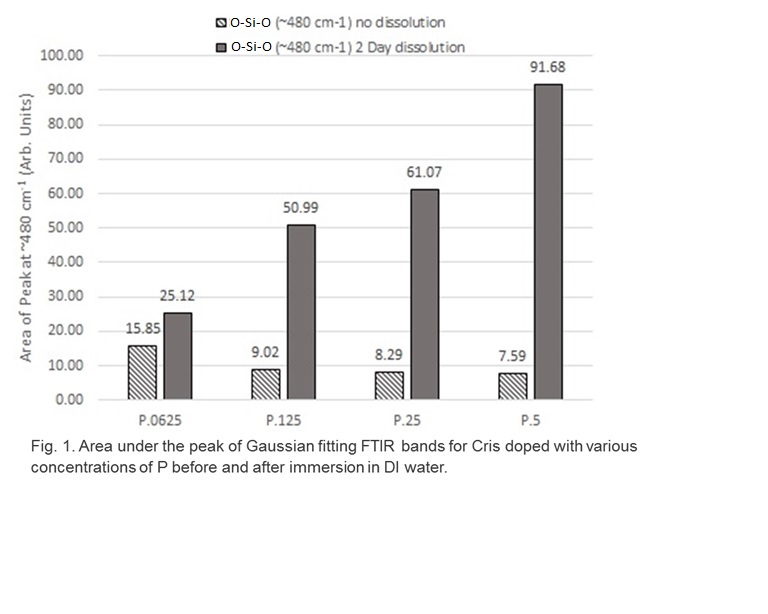
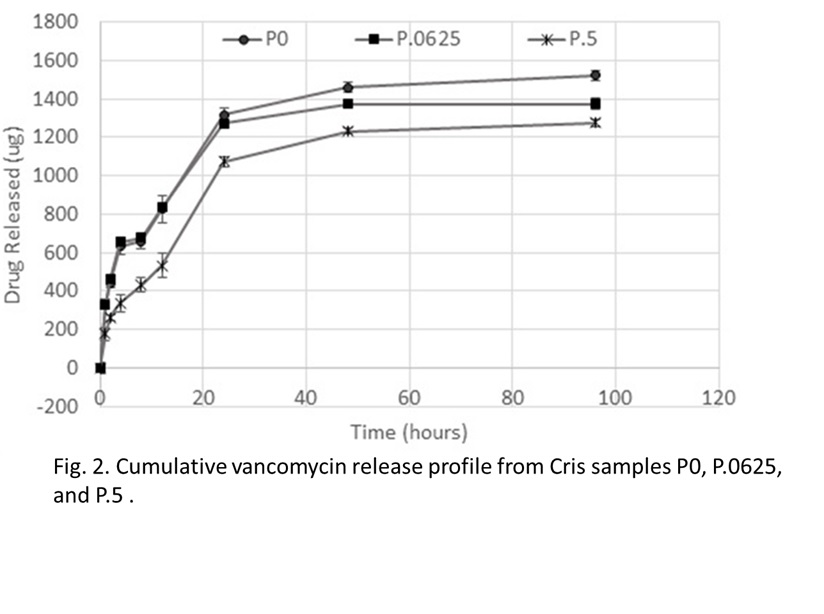
Discussion: Silicate phosphate ionic susbstitution was confirmed by XRD and FTIR. The release of P ions from the surface of Cris exposed active O-Si-O functional groups that enhanced drug binding as well as rapid release indicating phsyisorption. On the other hand, a slower drug release rate was observed as the P content increased in the matiertal surface indicating chemisorption.
Conclusions: Results from the present study indicate that it is possible to enhance the burst release stage of a bioceramic drug carrier by increasing the silicate functional groups. The sustained release profile can be engineered by controlling the phosphorus content of the bioceramic drug carrier.
References:
[1] Cosijns, An, Chris Vervaet, J. Luyten, S. Mullens, F. Siepmann, Luc Van Hoorebeke, Bert Masschaele, Veerle Cnudde, and Jean Paul Remon. "Porous hydroxyapatite tablets as carriers for low-dosed drugs." European Journal of Pharmaceutics and Biopharmaceutics 67, no. 2 (2007): 498-506.
[2] Ye, Feng, Haifeng Guo, Haijiao Zhang, and Xiulan He. "Polymeric micelle-templated synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system." Acta Biomaterialia 6, no. 6 (2010): 2212-2218.
[3] Gautier, H., C. Merle, J. L. Auget, and G. Daculsi. "Isostatic compression, a new process for incorporating vancomycin into biphasic calcium phosphate: comparison with a classical method." Biomaterials 21, no. 3 (2000): 243-249.
[4] Zamoume, Ouardia, Sophie Thibault, Gwenaël Regnié, Med Omar Mecherri, Marina Fiallo, and Patrick Sharrock. "Macroporous calcium phosphate ceramic implants for sustained drug delivery." Materials Science and Engineering: C 31, no. 7 (2011): 1352-1356.