Introduction: Proteins play critical and diverse roles in the normal function of the body, which makes them excellent candidates as therapeutics for diseases not easily treated with simple chemical drugs. However, proteins as a macromolecule present many stability issues, both during their manufacturing and subsequent usage in the body: their aggregation is often a problem and various host defense and immune mechanisms usually rapidly remove them from circulation. The solution is usually the addition of polyethylene glycol (PEG), or PEGylation, which provides bulk while conferring a protective layer. However, PEG adds another complexity, as multiple studies demonstrated a decrease in protein activity[1], as well as an apparent antigenicity of PEG once conjugated to a large carrier[2]. This translates to a compromise between the positive protection afforded by PEG and the increased immunogenicity of the final overall therapeutic product. In some systems, the former may not outweigh the problems introduced by the latter.
In contrast to the amphiphilic PEG, zwitterionic polymers based on naturally occurring betaines are entirely hydrophilic and confer the same protection without the complicating negative traits. These polymers as components of nanoparticles demonstrated excellent long-circulation abilities[3]-[5] and polymer-specific antibodies were absent, while anti-PEG antibodies were clearly detected and quantified[4],[5]. Furthermore, protein conjugates studied in vitro showed maintenance or improved activity profile of the underlying protein[6]. Combining these encouraging results, we hypothesize a protein conjugate of polyzwitterions will be non-immunogenic while sustaining native activity and prolonging circulation.
Materials and Methods: We used uricase as a model immunogenic enzyme and conjugated either PEG or zwitter polymer (PCB). The native enzyme and the two conjugates were administered at the same dose into rats weekly for 3 weeks. One week after the last injection blood was drawn for IgM detection and two weeks later for IgG detection via ELISA. Circulation profiles of the last injections were collected using serum uricase activity as a surrogate.
Results and Discussions: A typical antibody-mediated response starts with the generation of IgM within 1-2 weeks of initial immunogen encounter, which escalates to more rapid responses upon multiple exposures of the same agent. At this time, isotype switching occurs and IgG antibodies become the dominant isotype, which can achieve significant titer within days. Thus, within our study system, we expect elevated IgG generated in addition to IgM against uricase, but the presence of protective polymers on the bioconjugates may protect the underlying enzyme to some degree.
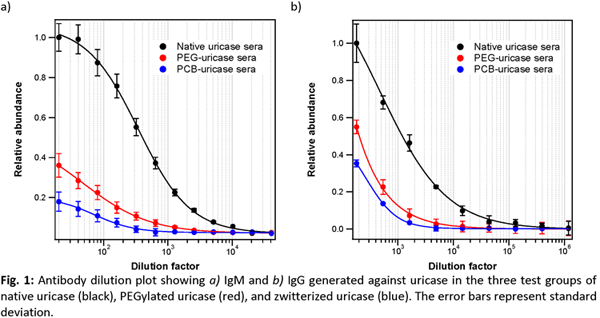
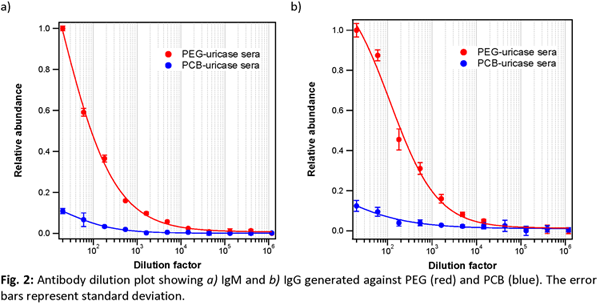
For both antibody isotypes, the animals receiving zwitterionic conjugates mounted the weakest response: 64x less IgM and 81x less IgG than the titer evoked by the native enzyme, as well as 8x less IgM and 3x less IgG than that elicited by PEGylated uricase (Fig. 1). As control, all baseline serum samples tested negative for either isotype. In addition to protein-specific immune response, previous work has highlighted the presence of polymer-specific antibodies, which continued to mediate immune clearance[2],[4],[5]. Fig. 2 shows the PEG IgM titer is 81x and the IgG titer is 243x that of the corresponding PCB titers. This correlates well with the circulation profile of the final dose: the elimination half-life of native uricase is the shortest at 6.2 h, followed by PEG conjugates at 25.6 h, while PCB conjugates has the longest half-life at 78.1 h. This is further reflected by the relative bioavailability: zwitterionic conjugate leads with 10.2x that of native uricase and 3.6x over PEG conjugates.
Conclusion: We demonstrate polyzwitterions as a stealth layer that protects the underlying protein therapeutic from host immune recognition without sacrificing enzymatic activity or inducing polymer-specific antibody production. The encouraging results presented here highlight the potential of these biocompatible polymers in the protection and delivery of exogenous and thus immunogenic biologics for human medicine.
This work was supported by DTRA and NIH.
References:
[1] Knop et al. Angew. Chem., Int. Ed., 2010, 49, 6288–6308
[2] Ishida et al. J. Control Release, 2007, 3, 349-355
[3] Zhang et al. ACS Nano, 2012, 6, 6681-6686
[4] Yang et al. Nano Today, 2014, 9, 10-16
[5] Zhang et al. Proc. Natl. Acad. Sci, 2015, 112, 12046-12051
[6] Keefe and Jiang. Nature Chem, 2012, 4, 59-63