Introduction: Cell fate is regulated by their interactions with its microenvironment as cells can sense and respond to the substrate's biochemical and biophysical cues including elasticity[1]. Therefore mechanical properties of scaffolds not only preserve scaffold's structural integrity and functionality, but also greatly influence cellular behavior in tissue engineering processes[2]. With the ability to form fibrous structures to mimic the extracellular matrix (ECM) of native tissue using collagen, a natural ECM component, it is desirable to measure and tune the elastic modulus of the collagen scaffold. Measurement of the elastic modulus of single fiber become essential as the overall mechanical properties of a fibrous scaffold is a function of the mechanical properties and assemblies of individual fibers[2]. Atomic force microscope (AFM) is a powerful tool for the determination of mechanical properties of nanomaterials due to its high spatial resolution and sensitivity to subnanonewton forces[3]. In this study we aim to measure the Young’s Modulus of electrospun collagen fibers using the multipoint bending testing with AFM.
Materials and Methods: Electrospinning of collagen was carried out in acetic acid, which has been proposed as a solvent with possible advantage of reducing collagen denaturation[4]. Effects of spinning parameters were explored to obtain fibers with desired morphology and sizes[5]. Electrospun collagen fibers (100-500nm) were deposited directly on custom-designed silicon wafers. AFM measurements were made using a multimode atomic force microscope with a Nanoscope IIIa controller. NP-S silicon nitride cantilevers with nominal spring constants of 0.12N/m were used for measurements. Samples were imaged in contact mode to locate isolated fibers suspended over gaps (Fig. 1a) and once a fiber is located, mechanical testing was performed by measuring force curves under force-volume mode. Igor Pro (WaveMetrics) with custom analysis routine[6] was used to analyze force plot data and obtain Young's modulus of single fiber.
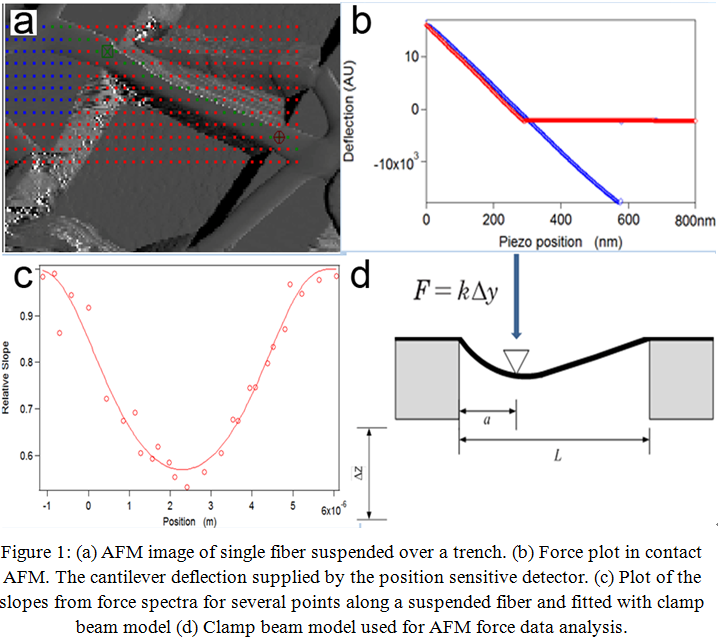
Results and Discussion: Topographic image of the fibers was used for points selection on fibers and substrate (Fig. 1a). The elastic modulus was determined using relative slopes of force spectra at various position along the suspended fibers (Fig. 1b). The deflection of the fibers at chosen points were analyzed with the analysis routine while the slopes of force plots were used to find the best fit to the data with the clamped beam model (Fig. 1c and 1d). The fitting result demonstrates that the clamped beam model is valid for the fiber and the Young's moduli of collagen fibers are approximately 1.3 - 10.5 GPa. Wenger et al.[7] demonstrated that rat tail collagen fibril have Young’s moduli in the range of 5 to 11.5 GPa. The obtained Young's modulus in this study is close to that of native rat tail collagen fibril. These results will be compared to those obtained when the fibers are exposed to water and cell culture media.
Conclusion: The calculated elastic modulus of the electrospun collagen fibers is close to that of the native fibril. By constructing scaffold with component from native ECM that possess appropriate mechanical properties to mimic the natural environment, the resultant scaffold can provide structure integrity and induce more normal and stable cellular functions.
Ontario Research Fund; Ontario Graduate Scholarship; Canada Foundation for Innovation; Axcelon Biopolymers Corporation
References:
[1] Discher, D.E.; Janmey, P., Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005, 310, 1139-1143
[2] Gobaa, S.; Hoehnel, S.; Roccio, M; Negro, A.; Kobel, S.; Lutolf, M.P. Artificial niche microarrays for probing single stem cell fate in high throughput. Nature Methods. 2011, 8, 949-955
[3] Kirmizis, D.; and Logothetidis, S. Atomic force microscopy probing in the measurement of cell mechanics. International Journal of Nanomedicine. 2010, 5, 137-145
[4] Chan, B.P.; Hui, T.Y.; Chan, O.C.M.; So, K.F.; Lu, W.; Cheung, K.M.C. Photochemical cross-linking for collagen-based scaffolds: a study on optical properties, mechanical properties, stability, and hematocompatibility. Tissue Engineering. 2007, 13, 73–85
[5] Li, Y.; Liu, J.; de Bruyn, J.R.; Wan, W.K. Optimization of the electrospinning process for core-shell fiber preparation. Journal of Biomaterials and Tissue Engineering. 2014, 4, 973-980
[6] Hudson, S.D.; Zhurov, V.; Grbic, V.; Grbic, M.; Hutter, J.L. Measurement of the elastic modulus of spider mite silk fibers using atomic force microscopy. Journal of Applied Physics. 2013, 113, 15437
[7] Wanger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical properties of collagen fibrils. Biophysical Journal. 2007, 93, 1255-1263