Introduction: The use of collagen-based biomaterials is highly versatile in many fields, such as medicine, dentistry and pharmacology[1]. The number of commercially available collagen-related products is rapidly increasing, and thence quality control methods are needed. Though hydroxyproline assay has been historically used as an estimate of collagen content[2], yet its measurement is a time-consuming and complicated process. Capillary electrophoresis (CE) is an advanced analytical technique suitable for analysis of proteins, peptides, and nucleic acids[3],[4]. Although several groups have reported separation and quantification of the cyanogen bromide peptides of collagen by CE[5], no information about quantification of total collagen was reported. We therefore developed a novel CE method to identify and quantify collagen in materials[6].
Materials and Methods: Porcine connective tissues (skin, intestines, cartilage, tendon, sponge bone, and compact bone) were purchased from traditional markets. CE was carried out using a PA 800plus system (Beckman Coulter, Fullerton, CA, USA).
Results: Different types of collagen showed the same migration time in the electropherogram of CE. Our method can effectively identify total collagen without interference from other proteins, such as ribonuclease A, albumin, cytochrome C, and lysozyme. The quantitation and detection limits of this method were 0.28 ng and 0.19 ng, respectively, and the collagen concentration and peak area showed a good linear relationship (r2 = 0.9995). To detect the actual collagen content of various tissue samples, this innovative approach was evaluated in parallel with traditional colorimetric analysis method. The values obtained from these two methods were statistically identical, demonstrating the accuracy and practicality of this innovative approach.
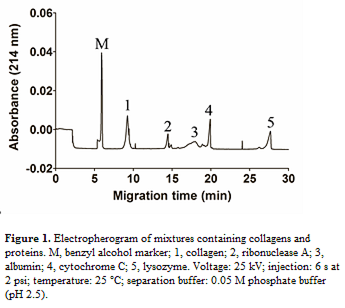
Discussion: To date, few methods have been developed to quantify total collagen. Among these methods, the sirius red assay is well established with a quantitation range of 5–40 µg/mL and was used by Taskiran et al. to quantify the total collagen in rabbit tendon[7]. Kliment et al. developed a novel colorimetric method for assessing the collagen content in murine pulmonary tissue[8]. In comparison with traditional hydroxyproline assay and the above colorimetric methods, our novel method using CE analysis has both chromatographic and quantification advantages, allowing us to evaluate the purity of collagen and quantify the amount at the same time.
Conclusion: In this study, we established a method to quantify total collagen or hydroxyproline from different tissues by CE. We further compared the CE results to the traditional hydroxyproline assay to evaluate the method. Our novel method effectively distinguishes between collagen and other tissue proteins and accurately quantifies the total collagen content. Moreover, this method can significantly shorten the experimental time and reduce sample quantity. This method can be used as a quality control analysis for collagen in the biomaterial as well as food sciences and cosmetic industry.
This work was supported in part by Ministry of Science and Technology of Taiwan, through Grants NSC-102-2622-M-006-002-CC2 and MOST-103-2622-M-006-002-CC2.
References:
[1] R. Khan, MH. Khan, J Indian Soc Periodontol 2013 17. 539–542.
[2] H. Stegemann, K. Stalder, Clinica chimica acta 1967, 18. 267-273.
[3] K. Swinney, D. J. Bornhop, Electrophoresis 2000, 21. 1239-1250.
[4] C. Prata, P. Bonnafous, N. Fraysse, M. Treilhou, V. Poinsot, F. Couderc, Electrophoresis 2001, 22. 4129-4138.
[5] I. Mikšík, P. Sedláková, K. Mikulíková, A. Eckhardt, Journal of Chromatography B 2006, 841. 3-13.
[6] Lynn L.H. Huang. Capillary electrophoresis method for quantitatively analyzing collagen and an assay kit for the same. Taiwan Patent I402503. PROC Patent 200910130395.6. US Patent 12/423,318.
[7] D. Taskiran, E. Taskiran, H. Yercan, F. Kutay, TURKISH JOURNAL OF MEDICAL SCIENCES 1999, 29. 7-10.
[8] C. R. Kliment, J. M. Englert, L. P. Crum, T. D. Oury, International journal of clinical and experimental pathology 2011, 4. 349-55.