Introduction: Recently porous biodegradable metals have been proposed as new materials for bone scaffolds [1] with magnesium as the most studied over the last 5 years [2]. Its porous structure was proven to play important role in cell growth, spreading and proliferation due to higher surface contact area and interconnectivity within the pores [3]. Meanwhile, porous iron is introduced more recently [4] where in vitro cytotoxicity assessments show positive effect toward the proliferation of mesenchymal and osteoblast cells [4],[5]. In this work we aim to further elaborate its potentiality as bone implant material via in vivo implantation study in femoral bone of rats and evaluation of the local and systemic body response.
Materials and Methods: This study was approved by Animal Care and Use Committee, Bogor Agricultural University (#6-2014 IPB). Implants (5 x 2 x 0.5 mm3) were prepared form pure porous iron (Alantum, Korea) with with pore size of 450 μm, 580 μm, and 800 μm. Sixty male Sprague Dawley rats (6-7 weeks age) were grouped based on the implant’s pore size and 1 sham group. Implants were inserted into the right femur bone defects. Local and systemic body responses were observed at day 7, 14, and 30 post-implantation (n=5). One mL of venous-tail blood was collected and analysed for cell blood count, biochemistry profile, and concentration of metal (Fe, Ca, P) ions. Peri-implant muscle samples were collected at near (±0.1 mm) and far (±5 mm) areas from implant for Fe ion concentration analysis. Radiograms were taken using portable digital computer radiography. Colorimetric analysis was done on retrieved implants and on opened area of implant-tissue contact using image analysis software. All data were statistically analyzed using one way ANOVA and Duncan test.
Results and Discussion: Fig. 1A presents evidence that porous iron implant only affect the percentage of neutrophil, lymphocyte, and monocyte due to the inflammation response against the implant [6]. The concentration of Fe and Ca ions was increase in the 1st weeks of implantation (Fig 1B) similar to our previous findings [7]. Fig. 1C shows the changes of kidney and liver enzyme function in the level of SGPT, SGOT, urea, and creatinine over time.
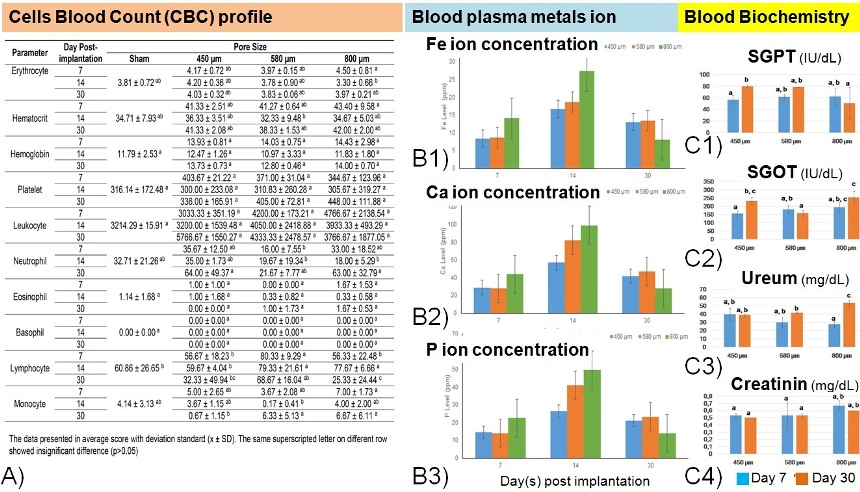
Fig. 1. Systemic blood profile analysis. (A) Cells blood count, (B1-3) Metal (Fe, Ca, and P) ion concentration, (C1-4) Liver and renal enzyme function biochemistry.
Fig. 2A shows radiograms with different degradation rate of the implants due to different pore size. However, there is no significant difference in term of peri-implant-bone radio-density and pore sizes (Fig. 2B). Iron ion concentration was found higher at the near peri-implant area and was decreasing over 30 days, except for the 800 μm group (Fig. 2C).
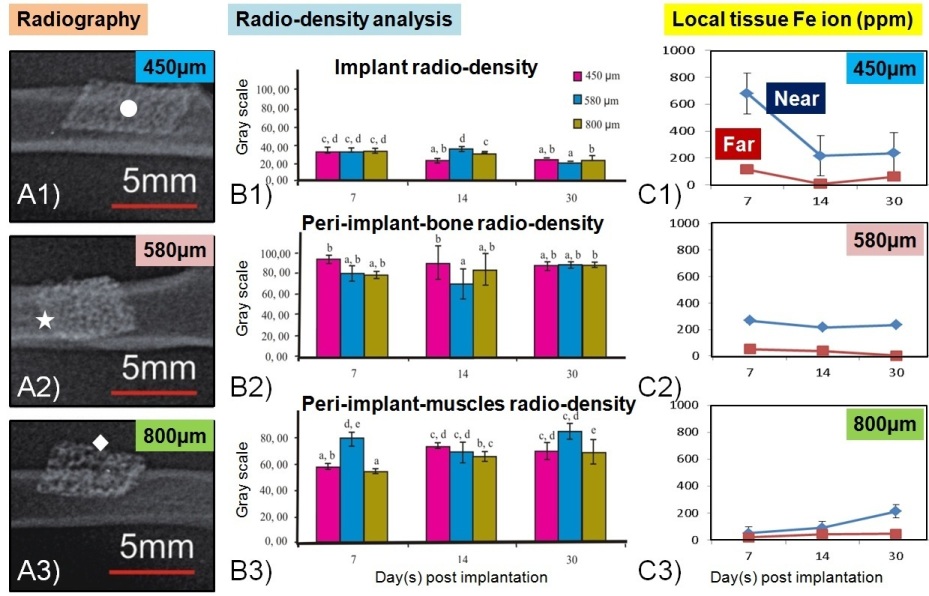
Fig. 2. Radiograms and local Fe ion analysis: (A1-3) Radiography at day 30, (B1-3) Radiodensity of implant, peri-implant-bone and peri-implant-muscles, (C1-3) Fe ions concentration at local tissue.
Fig. 3A shows that the colorimetric analysis on the opened peri-implant tissue and the retrieved implant has consistently with the radiographic analysis. The reddish-brown tissue intensity is higher for 450 μm implant group (Fig. 3B). This can be related to higher volume of iron in this group and the greater implant-tissue interaction area causing pronounce earlier degradation process[8].
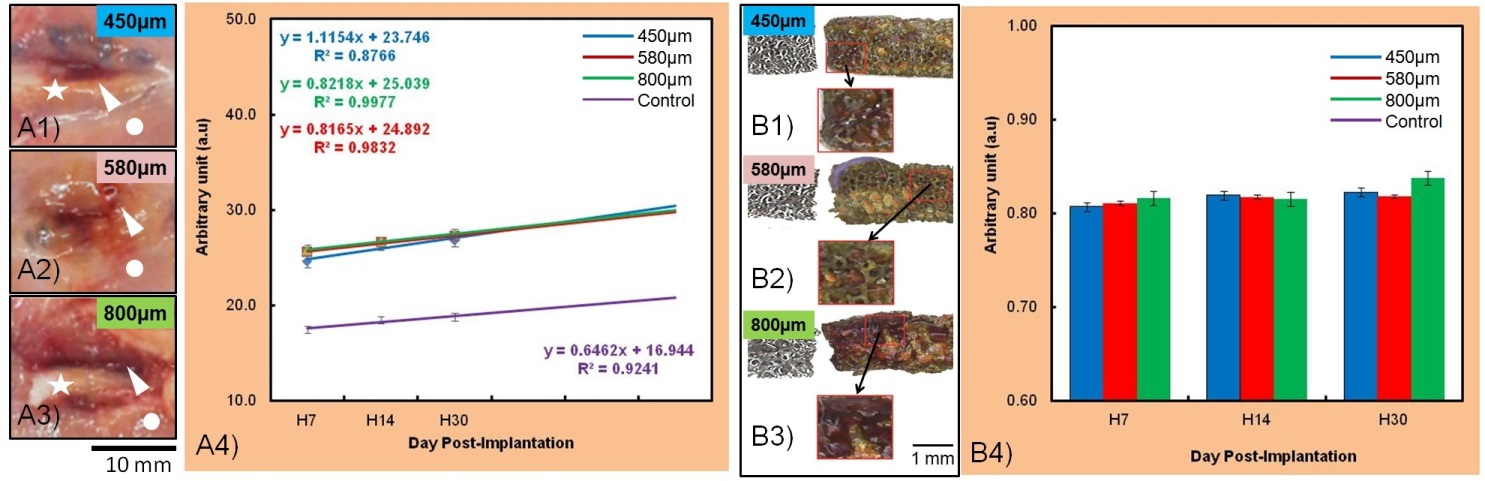
Fig. 3. Colorimetric image analysis of: (A1-4) Opened peri-implant tissue, (B1-4) retrieved implant and its corrosion product.
Conclusions: In general, the study provide evidences that the implantation of porous iron implants on the femoral bone of rats does not cause negative effect toward local and systemic biological responses of the animal.
The authors acknowledge Indonesian Ministry of Education and Culture-DGHE for grant No. 083/SP2H/PL/Dit.Litabmas/II/2015 and the collaboration with Alantum, Korea, and CHU de Quebec Research Center, Laval University.; This work in part of the international Indonesia-Canada collaboration between Laval University and Bogor Agricultural University.; The authors thank to Dr. Erwin, Dr. Sitaria Fransiska Siallagan, Dr. Tetty Barunawati Siagian, Dr. Frizky Amelia and Dr. Nindya Dwi Utami for the discussion.
References:
[1] A. H. Yusop, et al., International Journal of Biomaterials 2012;2012:641430.
[2] K. Lietaert, et al. Journal of Magnesium and Alloys 2013;1:303-311.
[3] G. Jiang, G. He, Materials Science and Engineering C 2014;3:317-320.
[4] R. Oriňáková, et al. International Journal of Electrochemical Science 2013;8:12451-12465.
[5] N. M. Daud, et al., Journal of Orthopedics Translation 2014;2:177-184.
[6] J. E. Schaffer, et al., Acta Biomaterialia 2013;9:8574–8584.
[7] M. F. Ulum, et al., European Cells and Materials 2013;26(Suppl.5):59.
[8] S. Bauer, et al., Progress in Materials Science 2013;58:261-326.