Introduction: Rapid Prototyping technologies are increasingly demonstrating the great potential for fabricating biocompatible 3D structures with precise control of the micro- and macro-scale characteristics[1],[2]. Despite it being the first commercialized RP technology, the use of Stereolithography (SL) in Tissue Engineering has not been significantly explored, probably due to the lack of commercially available implantable or biocompatible materials[3],[4]. The functionalization of oligomers such as ε-caprolactone (ε-CL), with unsaturated groups has been studied in order to obtain a biocompatible resin for stereolithography applications. In this work, a novel photocrosslinkable polycaprolactone (PCL) has been synthesized in order to realize refined 3D scaffolds by stereolithography technique.
Materials and Methods: Synthesis and functionalization of a photocrosslinkable precursor can be achieved in one step, by using Ring Opening Polymerisation (ROP) with a suitable combination of catalayst/initiator. A Sn-free catalyst has been used for the ROP of ε-CL with a high monomers conversion at room temperature, with 2-Hydroxyethyl vinyl ether (HEVE) as both initiator and photo-curable functional group. Following the synthesis of the vinyl-terminated PCL (VPLC), its reaction with fumaryl chloride (FuCl) results in chains containing photocrosslinkable vinyl units at both ends and in the middle of the chain in order to obtain a divinyl-fumarate-PCL (VPCLF).
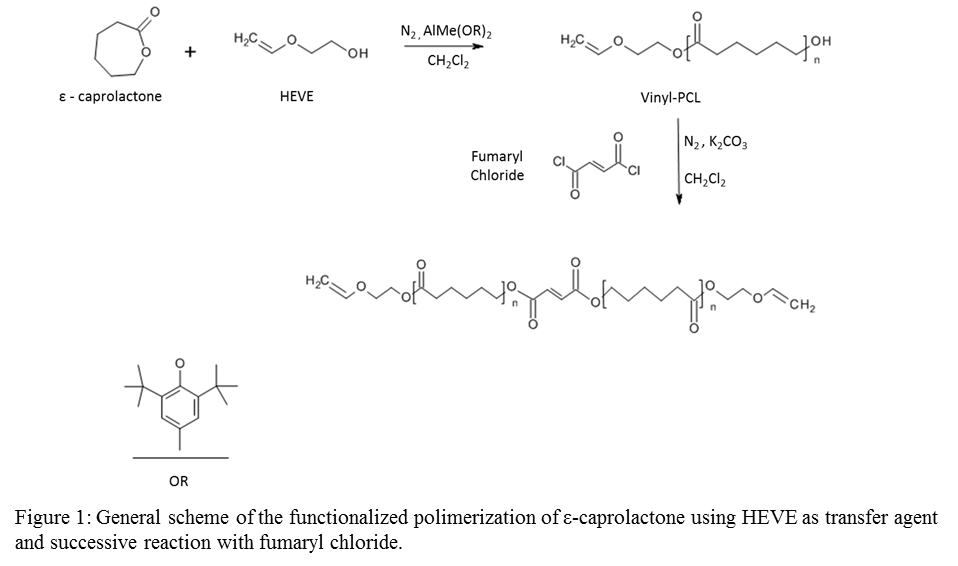
Networks were formed from VPCLF by UV irradiation (365 nm) using Lucirin TPO as a biocompatible initiator, Orasol Orange Gas dye and N-Vinyl-2-pyrrolidone (NVP) as reactive solvent. Mathematically defined porous structures with gyroid and diamond[5],[6] architecture were prepared using the VPCLF/NVP resin.
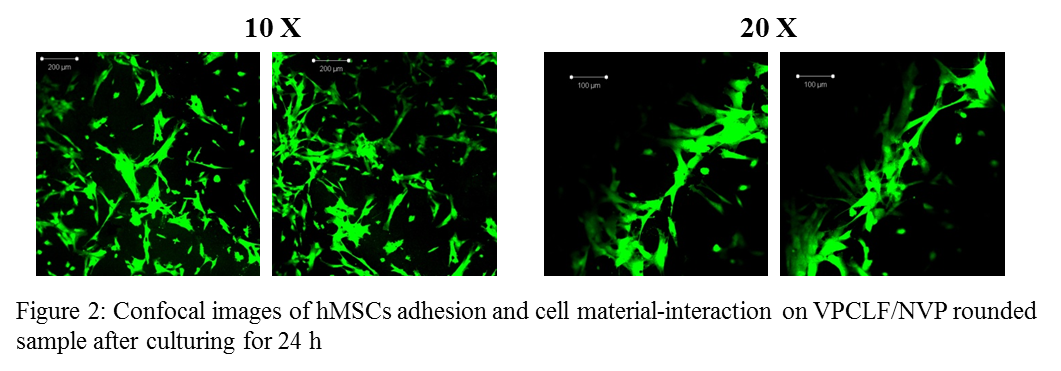
Results and Discussion: The resulting polymer was characterized by its specific molecular weights, functional end groups and transition temperatures using several techniques including: Nuclear Magnetic Resonance (NMR), Fourier Transformed Infrared Spectroscopy (FTIR), Differential Scanning PhotoCalorimetry (DPC) and Differential Scanning Calorimetry (DSC). Photo crosslinked disk-shaped specimens (diameter 6 mm) of VPCLF/NVP were seeded with human mesenchymal stem cells (hMSCs) to assess material’s biocompatibility. Cell morphology and cell spreading pattern interaction onto VPCLF/NVP specimens were evaluated by confocal laser scanning microscopy.
SEM images and micro tomography (µ-CT) reconstruction show structures with an open pore architecture fully interconnected, allowing cell seeding and proliferation.
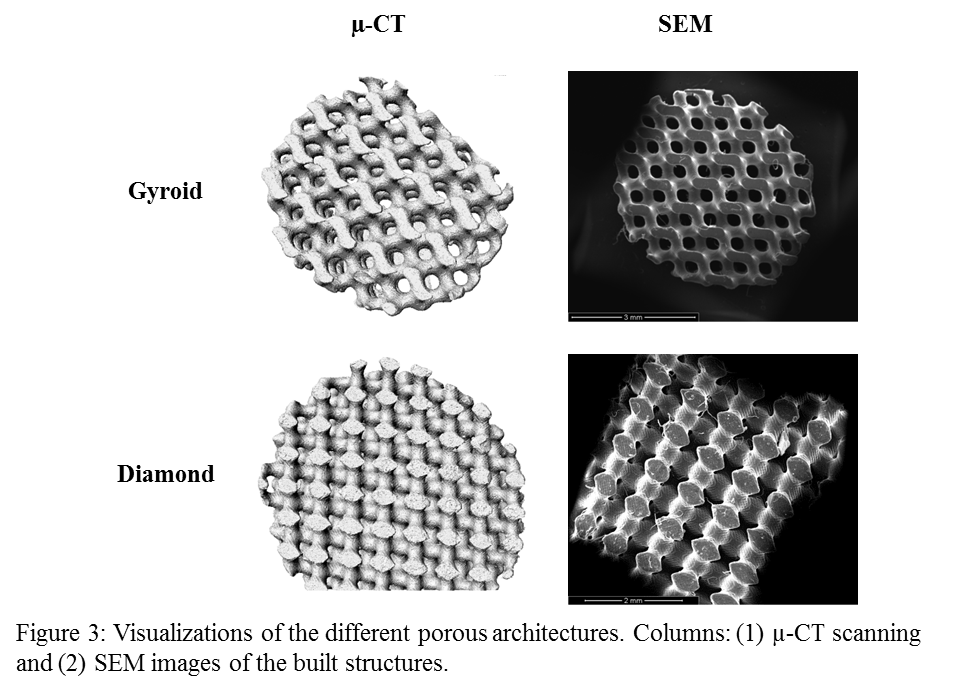
Conclusions: A resin based on VPCLF macromers and a NVP diluent was developed and applied in stereolithography. Biological results indicate good cell adhesion for the VPCLF/NVP networks demonstrating that materials appeared not to be cytotoxic. Moreover designed porous scaffolds with diamond and gyroid architectures were prepared and characterized. Results proved that it is possible to obtain mathematically refined scaffolds with a homogeneous micro architecture by stereolithography technique using VPCLF/NVP photocurable resin.
The authors would like to thank the MAGISTER Research Project (NMP3-LA-2008-214685) co-funded by the European Community's under the FP7-Cooperation Programme for providing financial support to this project.
References:
[1] K. Kim, A. Yeatts, D. Dean and J. P. Fisher, “Stereolithographic Bone Scaffold Design Parameters: Osteogenic Differentiation and Signal Expression”, Tissue Engineering: part B. Vol 16, Oct 2010.
[2] A. Gloria, T. Russo, R. De Santis and L. Ambrosio, “3D fiber deposition technique to make multifunctional and tailor-made scaffolds for tissue engineering applications”, Journal of Applied Biomaterials and Biomechanics. Vol. 7, 2009
[3] F.P.W. Melchels, J. Feijen and D. W. Grijpma, “A poly(d,l-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography”, Biomaterials. Vol. 30, Aug. 2009
[4] A. Ronca, L. Ambrosio and D.W. Grijpma, “Preparation of designed poly(d,l-lactide)/nanosized hydroxyapatite composite structures by stereolithography”, Acta Biomaterialia. Vol.9, Apr. 2013
[5] P.J.F. Gandy and J. Klinowski, “Exact computation of the triply periodic G (`Gyroid') minimal surface”, Chemical Physics Letters. Vol. 321, May 2000
[6] P.J.F. Gandy, D. Cvijović, A.L. Mackay and J. Klinowski, “Exact computation of the triply periodic D (`diamond') minimal surface”, Chemical Physics Letters Vol. 314, Dec. 1999