Introduction: Silk fibroin (SF) in the form of fibers is a kind of well-liked biomaterial for engineering load-bearing biological tissues. Electrospinning has been known to produce ultrafine SF fibers in nano/micro-scale fineness that highly mimics the ultrastructure of extracellular matrix in many tissues. However, ultrafine SF fibers from conventional electrospinning usually are of low mechanical strength and poor degree of orientation. To address the noted problems, this study attempts to fabricate highly aligned ultrafine SF fibers through a novel stable jet electrospinning (SJES) approach[1]-[3], from which their physicochemical and biological properties can be evaluated for potential tissue scaffolding applications.
Materials and Methods: Poly(ethylene oxide) (PEO, 0-14 wt%) was employed to formulate the spinning dope of SF so as to generate a long stable jet during the SJES. The as-electrospun SF fibers were subsequently treated in a methanol solution (90%) for 10 min (termed as M-SF/PEO). Thereafter, PEO in the M-SF/PEO fibers were extracted by being immersed in distilled water for 2 days (termed as W-M-SF/PEO). These SF fibers were thoroughly characterized by different techniques. Finally, cytocompatibility of these ultrafine SF fibers was evaluated using murine iPSC-derived MSCs (i.e., iPSC-MSCs) by Cell Counting Kit-8 (CCK-8) assay.
Results and Discussion: Introduction of PEO into SF is beneficial to eliminate the occurrence of jet bending instability so that a very long linear jet can be obtained (Fig. 1A). The formulation SF/PEO (SF:PEO = 88:12) appeared to be the optimal solution system that gave rise to highly aligned ultrafine SF fibers: more than 90% of fibers with an averaged diameter of ~1200 nm oriented unidirectionally (angle variation < 1°) (Fig. 1B,C). XRD patterns of the SF fibers suggest configuration transformation from amorphous to beta-sheet after methanol treatment (Fig. 1D). The disappearance of absorption peak of PEO at 1101 cm-1 (C-O-C) in W-M-SF/PEO fibers confirmed the effectiveness in extracting PEO by soaking in water (Fig. 1E). Compared to random SF fibers, the M-SF/PEO fibers showed higher breaking tenacity (53.3 ± 14.0 MPa, increased by 53.9-fold) and Young's modulus (2803.1 ± 435.0 MPa, increased by 52.85-fold) (Fig. 1F), whereas the W-M-SF/PEO fibers became mechanically weaker but more hydrophilic after water treatment (Fig. 1G). These SF fibers supported the iPSC-MSCs to adhere and grow in a manner of orienting along the fiber axis (images not shown) and the cell proliferation was ameliorated on the W-M-SF/PEO fibers (Fig. 1H).
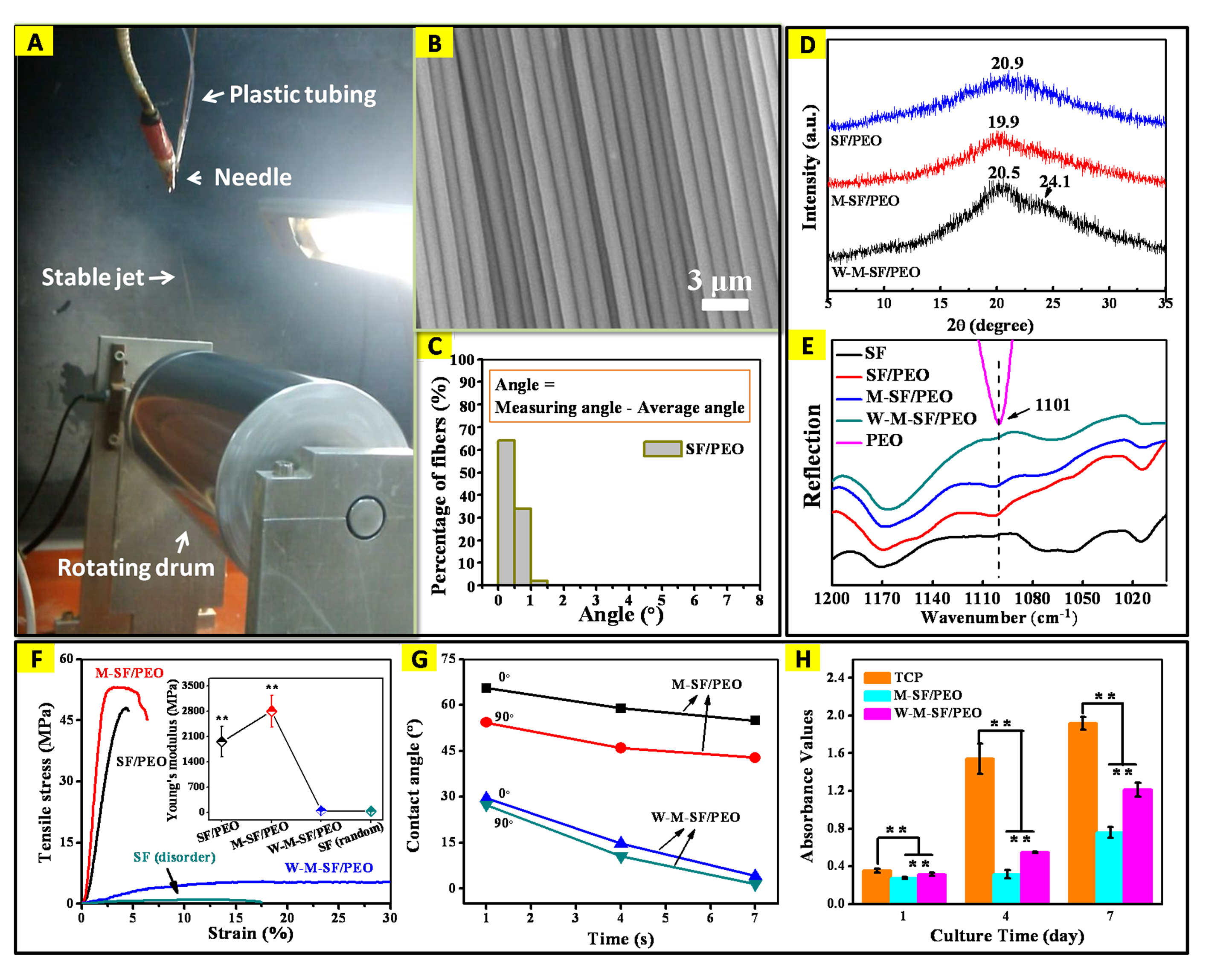
Fig.1. (A) Actual process of SJES in collecting aligned SF fibers. (B) SEM images and (C) angular histograms of the aligned SF fibers prepared with the following variables: applied voltage 8 kV, feed rate 0.2 mL/h, rotating speed 600 rpm and collecting distance 22 cm. (D) XRD patterns and (E) IR spectra of the aligned SF/PEO, M-SF/PEO and W-M-SF/PEO fibers and raw components of SF and PEO. (F) Typical tensile stress-strain curves of SF-based fibers; inset shows the Young's modulus data. (G) Anisotropic wettability by measuring water contact angles of the different fibers in alignment. (H) Cell proliferation of the iPSC-MSCs on TCP, M-SF/PEO and W-M-SF/PEO fibers after culturing for 1, 4 and 7days. **p < 0.01.
Conclusions: This study demonstrated that ultrafine SF fibers could be successfully produced via the SJES method. With the high degree of fiber orientation, impressive mechanical properties, and good cytocompatibility, our electrospun SF fibers may be considered as high performance fibers for engineering load-bearing tissues (e.g., tendon, ligament, and blood vessel) in future.
This work was partially supported by the National Natural Science Foundation of China (51073032, 31570969), the Natural Science Foundation of Shanghai (15ZR1400500), the Key Basic Research Foundation of Shanghai Committee of Science and Technology (14JC1490100), and the Fundamental Research Funds for the Central Universities by the Ministry of Education of China (2232013D3-13, 15D110538).
References:
[1] Yuan, H., et al., Stable jet electrospinning for easy fabrication of aligned ultrafine fibers. Journal of Materials Chemistry, 2012. 22(37): p. 19634-19638.
[2] Yuan, H., et al., Aligned Ultrafine Chitosan Fibers from Stable Jet Electrospinning. Acta Polymerica Sinica, 2014(1): p. 131-140.
[3] Zhou, Q., et al., Implication of stable jet length in electrospinning for collecting well-aligned ultrafine PLLA fibers. Polymer, 2013. 54(25): p. 6867-6876.